
���� ��1������ͭ�����ڿ�������ȴ�����տ����е�ˮ�������γ�����ͭ���壻
��2��ͨ��������Ǻ���ʵ�飬����ȷ������ͭ������ˮ���ʧȥ��
��3��������������Ϊm������������ͭ���������Ϊm1��������ͭ���������Ϊm1-m�����Ⱥ������������ˮ����ͭ������Ϊm2����ᾧˮ������Ϊm1-m2���ݴ˼���x��
��4��A�������к��м��Ȳ��ӷ������ʣ���ᵼ�²����ˮ������ƫС��
B�������к��м����ӷ������ʣ���ᵼ�²����ˮ������ƫ��
C������ǰ�������в�����ˮ����ᵼ�²����ˮ������ƫС��
D��ʵ��ǰ����δ��ȫ�����ᵼ�²����ˮ������ƫ��
E�����������ˮ����ȫ��ᵼ�²����ˮ������ƫС��
F������ʱ�о��彦������ᵼ�²����ˮ������ƫ��
��� �⣺��1������ͭ�����ڿ�������ȴ�����տ����е�ˮ�������γ�����ͭ���壬Ӱ��ʵ��Ľ������ʹ��õĽᾧˮ�ĺ���ƫС��
�ʴ�Ϊ���ڿ�������ȴ�����տ����е�ˮ��������ʹ��õĽᾧˮ�ĺ���ƫС��
��2��ͨ��������Ǻ���ʵ�飬����ȷ������ͭ������ˮ���ʧȥ��
�ʴ�Ϊ��ȷ������ͭ������ˮ���ʧȥ��
��3��������������Ϊm������������ͭ���������Ϊm1��������ͭ���������Ϊm1-m�����Ⱥ������������ˮ����ͭ������Ϊm2����ᾧˮ������Ϊm1-m2������$\frac{18x}{160+18x}$=$\frac{m{\;}_{1}-{{m}_{2}}_{\;}}{m{\;}_{1}-m}$�ɵ�x=$\frac{80��{m}_{1}-{m}_{2}��}{9��{m}_{1}-{m}_{\;}��}$��
�ʴ�Ϊ��$\frac{80��{m}_{1}-{m}_{2}��}{9��{m}_{1}-{m}_{\;}��}$��
��4��A�������к��м��Ȳ��ӷ������ʣ���ᵼ�²����ˮ������ƫС�����Ի�ʹ���Խ��ƫ�ͣ�
B�������к��м����ӷ������ʣ���ᵼ�²����ˮ������ƫ�����Ի�ʹ���Խ��ƫ�ߣ�
C������ǰ�������в�����ˮ����ᵼ�²����ˮ������ƫС�����Ի�ʹ���Խ��ƫ�ͣ�
D��ʵ��ǰ����δ��ȫ�����ᵼ�²����ˮ������ƫ�����Ի�ʹ���Խ��ƫ�ߣ�
E�����������ˮ����ȫ��ᵼ�²����ˮ������ƫС�����Ի�ʹ���Խ��ƫ�ͣ�
F������ʱ�о��彦������ᵼ�²����ˮ������ƫ�����Ի�ʹ���Խ��ƫ�ߣ�
��ѡBDF��
���� ���⿼��������ͭ�����нᾧˮ�����IJⶨ����Ŀ�ѶȲ���ע�����ղⶨ����ͭ����ᾧˮ�����ķ�������ȷʵ������к��س��������弰����ͭ�����нᾧˮ�ļ��㷽����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=4.3��CH3COOH��CH3COONa�����Һ�У�c��Na+����c��CH3COO-�� | |
| B�� | Ũ��Ϊ0.2mol/L��CH3COOH��Һ��ũ��Ϊ0.1mol/L��NaOH��Һ�������Ϻ�c��CH3COO-��-c��CH3COOH��=2[c��H+��-c��OH-��] | |
| C�� | ������Һ������ˮϡ�ͣ�$\frac{c��C{H}_{3}COOH��}{{c}^{2}��{H}^{+}��}$�������� | |
| D�� | amol/LCH3COOH��Һ��bmol/LNaOH��Һ�������ϣ�������Һ��c��Na+����c��CH3COO-������һ����a��b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ܶȱȿ���С | B�� | ����ɫ��ζ������ | ||
| C�� | KOH��Һ������̿������������ | D�� | ��ʹ�������ɫ������ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | SO2��NO2��CO2���������������� | |
| B�� | ����ͺ�һ�ȼ��鶼�������� | |
| C�� | ���������ﶼ�ǽ��������� | |
| D�� | ��ˮ��Һ���ܵ����H+�Ļ����ﶼ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
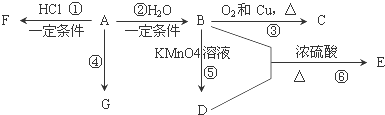
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ǿ�������ˮ��һ����ȫ���ܽ� | |
| B�� | Cu�ܵ��磬���Cu�ǵ���� | |
| C�� | ���������Һ�ĵ����Կ��ܱ�ǿ�������Һ�ĵ�����ǿ | |
| D�� | NaCl�ǵ���ʣ���NaCl�����ܵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
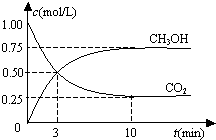
| A�� | �ӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����Ϊ0.075 mol/��L•min�� | |
| B�� | �ӷ�Ӧ��ʼ��ƽ�⣬������ת����Ϊ75% | |
| C�� | ���ܱ����������Ϊ1L | |
| D�� | ���¶��£���Ӧ��ƽ�ⳣ����ֵΪ$\frac{16}{3}$ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ��� | SiO2 | C | Na2O | K2O | Al2O3 | Fe2O3 |
| �������� | 59.20 | 38.80 | 0.25 | 0.50 | 0.64 | 0.16 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������������Һȥ���������������Ĥ��Al2O3+2OH-�T2AlO2-+H2O | |
| B�� | ͭƬ����ϡ���������ɫ���壺Cu+4H++2NO3-�TCu2++2NO2��+2H2O | |
| C�� | �����������ڿ����б��ʣ�2Fe��OH��2+O2+2H2O�T2Fe��OH��3 | |
| D�� | ̼������Һ�ʼ��ԣ�CO32-+2H2O?H2CO3+2OH- |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com