
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵڰ��� |
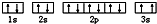 ��ͬѧ�����ĵ����Ų�ͼΥ����
��ͬѧ�����ĵ����Ų�ͼΥ����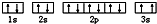 ����ͬѧ�����ĵ����Ų�ͼ��3s�ܼ���2����������������ͬ��Υ��������ԭ����
����ͬѧ�����ĵ����Ų�ͼ��3s�ܼ���2����������������ͬ��Υ��������ԭ����


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ��ǣ� I1=738kJ/mol I2=1451kJ/mol I3=7733kJ/mol I4=10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵ����� |


 ��ͬѧ�����ĵ����Ų�ͼΥ����
��ͬѧ�����ĵ����Ų�ͼΥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�츣��ʡ�߶���ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
( 12��)������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
|
AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
|
BԪ��ԭ�ӵĺ���p��������s��������1 |
|
Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
|
Dԭ�Ӻ�������p���ȫ������� |
|
EԪ�ص������������������IJ�Ϊ4 |
|
F��ǰ�������е縺����С��Ԫ�� |
|
G�����ڱ��ĵڰ��� |
��1����֪BA5 Ϊ���ӻ�������� �� ���������ɵģ��ѧ���ţ���
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��4��G�
�壬G3+�۵����Ų�ʽΪ
��GE3������Ϊ���壬�۵� ���е�
���е� ����
���� ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ����ռ乹��Ϊ ��
��6��ǰ����������Fͬһ�������Ԫ�طֱ���EԪ���γɻ�����侧����۵��ɸߵ��͵�����˳��Ϊ��д��ѧʽ�� ��ԭ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ԫ�أ�����A��B��C��D��EΪ����������Ԫ�أ�F��GΪ��������Ԫ�أ����ǵ�ԭ����������������ش����⡣
| AԪ�صĺ���������͵��Ӳ�����ȣ�Ҳ����������ḻ��Ԫ�� |
| BԪ��ԭ�ӵĺ���p��������s��������1 |
| Cԭ�ӵĵ�һ�����ĵ����ֱܷ���: I1=738kJ/mol I2 = 1451 kJ/mol I3 = 7733kJ/mol I4 = 10540kJ/mol |
| Dԭ�Ӻ�������p���ȫ������� |
| EԪ�ص������������������IJ�Ϊ4 |
| F��ǰ�������е縺����С��Ԫ�� |
| G�����ڱ��ĵڰ��� |
��1����֪BA5 Ϊ���ӻ�������� �� ���������ɵģ��ѧ���ţ���
��2��B��̬ԭ����������ߵĵ��ӣ���������ڿռ��� ������ԭ�ӹ���� �Ρ�
![]() ��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��3��ijͬѧ����������Ϣ���ƶ�C��̬ԭ�ӵĺ�������Ų�Ϊ��
��ͬѧ�����ĵ����Ų�ͼΥ���� ��
��4��Gλ�� �壬G3+�۵����Ų�ʽΪ ��GE3������Ϊ���壬�۵�![]() ���е�
���е�![]() ����
����![]() ����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��-ks5u
����������������ˮ��Ҳ���������ѡ���ͪ���л��ܼ����ݴ��ж�GE3�ľ�������Ϊ________________��-ks5u
��5��DE3 ����ԭ�ӵ��ӻ���ʽΪ ����ռ乹��Ϊ ��
��6��ǰ����������Fͬһ�������Ԫ�طֱ���EԪ���γɻ�����侧����۵��ɸߵ��͵�����˳��Ϊ��д��ѧʽ�� ��ԭ����
��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com