
| A�� | ���º��ݣ�����1����1 mol N2+3 molH2������2����2 mol NH3 | |
| B�� | ���º�ѹ������1����1 mol N2+3 molH2������2����2 mol NH3 | |
| C�� | ���º��ݣ�����1����1 mol N2+3 molH2������2����3 mol NH3 | |
| D�� | ���º�ѹ������1����1 mol N2+3 molH2������2����3 mol NH3 |
���� A���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º����£��Ӳ���2ת������ߣ��ɵ�1 mol N2+3 mol H2�������1��Ӧ����ֵ����ʵ�����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�����ʵ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ2��1-b��mol���ݴ˼����жϣ�
B���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º�ѹ�£��Ӳ���2ת������ߣ��ɵ�1 mol N2+3 mol H2�������1��Ӧ����ֵ����ʵ���֮����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�ĺ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ2��1-b��mol���ܵ����ʵ���Ϊ��2+2b��mol�����ݺ�����ͬ�����жϣ�
C���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º����£��Ӳ���2ת������ߣ��ɵ�1.5 mol N2+4.5mol H2�������1��Ӧ����ֵ����ʵ�������ȣ������1���ǵ�Чƽ�⣬�ɵ�ЧΪ�ڲ���2�ڲ���1�Ļ���������ѹǿ��ƽ��ʱNH3�����������ʵ����Ȳ���1���������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ3��1-b��mol���ܵ����ʵ���Ϊ��3+3b��mol������ƽ���ǰ����ĺ��������жϣ�
D���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º�ѹ�£��Ӳ���2ת������ߣ��ɵ�1.5mol N2+4.5mol H2�������1��Ӧ����ֵ����ʵ���֮����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�ĺ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ3��1-b��mol���ܵ����ʵ���Ϊ��3+3b��mol�����ݺ�����ͬ�����жϣ�
��� �⣺A���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º����£��Ӳ���2ת������ߣ��ɵ�1 mol N2+3 mol H2�������1��Ӧ����ֵ����ʵ�����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�����ʵ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ2��1-b��mol��������2amol=2��1-b��mol��������a+b=1����A�����ϣ�
B���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º�ѹ�£��Ӳ���2ת������ߣ��ɵ�1 mol N2+3 mol H2�������1��Ӧ����ֵ����ʵ���֮����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�ĺ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ2��1-b��mol���ܵ����ʵ���Ϊ��2+2b��mol������$\frac{2a}{4+2a}$=$\frac{2��1-b��}{2+2b}$��������a+b=1����B�����ϣ�
C���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º����£��Ӳ���2ת������ߣ��ɵ�1.5 mol N2+4.5mol H2�������1��Ӧ����ֵ����ʵ�������ȣ������1���ǵ�Чƽ�⣬�ɵ�ЧΪ�ڲ���2�ڲ���1�Ļ���������ѹǿ��ƽ��ʱNH3�����������ʵ����Ȳ���1���������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ3��1-b��mol���ܵ����ʵ���Ϊ��3+3b��mol������$\frac{2a}{4-2a}$��$\frac{3��1-b��}{3+3b}$��������a+b��1����C���ϣ�
D���÷�Ӧ����Ӧ������������ٵķ�Ӧ�����º�ѹ�£��Ӳ���2ת������ߣ��ɵ�1.5mol N2+4.5mol H2�������1��Ӧ����ֵ����ʵ���֮����ȣ������1Ϊ��Чƽ�⣬ƽ��ʱNH3�ĺ�����ͬ�������1��N2��ת����Ϊa���ɷ���ʽ��֪ƽ��ʱNH3Ϊ2amol��ƽ��ʱ�ܵ����ʵ���Ϊ��4+2a��mol������2�� NH3��ת����Ϊb��ƽ��ʱNH3Ϊ3��1-b��mol���ܵ����ʵ���Ϊ��3+3b��mol����������$\frac{2a}{4+2a}$=$\frac{3��1-b��}{3+3b}$��������a+b=1����D�����ϣ�
��ѡC��
���� ���⿼�黯ѧƽ����㡢ƽ���ƶ�����Чƽ��ȣ��Ѷ��еȣ������Чƽ����ɡ��ж�ƽ���ƶ��ǽ���Ĺؼ���


 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

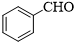 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ���� | c��HA��/mol•L-1 | c��NaOH��/mol•L-1 | ��Ӧ����ҺpH |
| �� | 0.1 | 0.1 | 9 |
| �� | c1 | 0.2 | 7 |
| A�� | �ף���Ӧ����Һ�� c��Na+����c��A-����c��OH-����c��H+�� | |
| B�� | �ң���Ӧ����Һ�� c��Na+��=c��HA��+c��A-�� | |
| C�� | ������ˮ���������c��H+��=1��10-9 mol•L-1 | |
| D�� | ����c1һ������0.2 mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | 5 | B�� | 3 | C�� | 2 | D�� | 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ij���ܵ�ص�ԭ������ͼ��ʾ����Һ��c��H+��=2.0mol/L��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ۵ĵ缫��ӦΪV3++e-=V2+������������ȷ���ǣ�������
ij���ܵ�ص�ԭ������ͼ��ʾ����Һ��c��H+��=2.0mol/L��������ΪSO42-��a��b��Ϊ���Ե缫�����ʱ�Ҳ۵ĵ缫��ӦΪV3++e-=V2+������������ȷ���ǣ�������| A�� | �ŵ�ʱ�����·�ĵ�����a������b�� | |
| B�� | �ŵ�ʱ����Һ��H+����������Ҳ� | |
| C�� | ���ʱ��a���ķ�ӦʽΪVO2+-e-+H2O=VO2++2H+ | |
| D�� | �������Һ��ɫ����ɫ��Ϊ��ɫʱ�����������ת����ʽΪ��ѧ��ת��Ϊ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | BaSO4���ۻ�״̬�µ���ı���ԭ�������д��������ƶ������� | |
| B�� | �Ȼ���������ˮ������ˮ��Һ������ | |
| C�� | Һ̬HCl�к��������ƶ���Cl- | |
| D�� | �����ǵ������ͨ������������������ƶ������ӵĹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
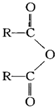 $\stackrel{H_{2}O}{��}$2RCOOH������R��������
$\stackrel{H_{2}O}{��}$2RCOOH������R��������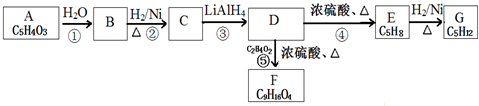
 ��CH2=C��CH3��CH=CH2��
��CH2=C��CH3��CH=CH2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �ۢ� | C�� | �ڢ� | D�� | �ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com