
����Ŀ����������10�����ʵ������գ�
��O2 ��H2 �� He ��K2O2 ��Ba(OH)2 ��CH4 ��Al ��NaF ��NH3����I2��
��1�������ɹ��ۼ��γɵĵ�����__________���������Ӽ����м��Լ�����_____���������ӻ��������_______________.�������ǹ�̬���ӵ���____________��
��2����NH3 �ĵ���ʽ��________________
�������������ܽ�������н�����ȷ����______________
�����ӻ�����ֻ�������Ӽ�
��ֻҪ�����Ӽ��Ļ�����������ӻ�����
��ֻҪ�й��ۼ������ʾ��ǹ��ۻ�����
�����ۻ�����ֻ�й��ۼ�
�����Լ������ܴ��������ӻ�������
������Ԫ����ǽ���Ԫ��֮��ֻ���γ����Ӽ�
���ǽ���Ԫ����ǽ���Ԫ��֮��ֻ���γɹ��ۼ���
���𰸡� �٢ڢ� �� �ܢݢ� 10 ![]() �ڢܢ�
�ڢܢ�
����������1���ɹ��ۼ��γɵĵ���Ӧ���Ƕ�ԭ�ӵ��ʷ��ӣ��٢ڢ���Ϊ�ɹ��ۼ��γɵĵ��ʣ��������Ӽ����м��Լ����������������Ӽ��ľ�Ϊ���ӻ�����������ӻ���������ܢݢ����������ǹ�̬���ӵ�������
��1��һ����˵�����ý����ͻ��÷ǽ�������֮�����γ����Ӽ�����ͬ�ǽ���Ԫ��֮�����γɼ��Լ���ͬ�ַǽ���Ԫ��֮�����γɷǼ��Լ���ֻ�зǽ���Ԫ����ɵ����ӻ�����������泥��������Ӽ����м��Լ���������李��������ƣ��ʴ�Ϊ�������ۢ���
��2��NH3�ǹ��ۻ���������ʽ��![]() ��
��
��3�����������Ӽ��Ļ����������ӻ�������ӻ����ﺬ�����Ӽ���Ҳ���ܺ��м��Լ���Ǽ��Լ�����KOH��Na2O2���ʴ������������Ӽ��Ļ����������ӻ�����������ӻ�������һ���������Ӽ�������ȷ����ֻ�����ۼ��Ļ������ǹ��ۻ�������й��ۼ��IJ�һ���ǻ�������������ʴ�����ֻ�����ۼ��Ļ������ǹ��ۻ�������ۻ�����ֻ�й��ۼ�������ȷ�������ӻ������п��ܺ��м��Լ�����KOH�к���O-H���Լ����ʴ���������Ԫ����ǽ���Ԫ��֮��������γɹ��ۼ������Ȼ������ɽ���Ԫ����ǽ���Ԫ���γɵĹ��ۼ����ʴ������ɷǽ���Ԫ����ɵĻ����ﲻһ���ǹ��ۻ�������������ӻ��������Σ��������Ӽ�������ȷ���ʴ�Ϊ���ڢܢ���


 С�����ϵ�д�
С�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����и������ʿ��÷�Һ©���������ȷ����� �� ��
���Ҵ������� ����������Һ��������Һ �۱���ʳ��ˮ �ܱ��ͱ��� �������������Ҵ� ���������ʹ�����Һ
A. �ۢ� B. �٢ۢܢ� C. �٢ڢܢ� D. �ۢݢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ʵ�������ܶ�Ϊ1.18g/mL����������Ϊ36.5%Ũ��������250mL0.1mol/L��������Һ,��ղ���ش��������⣺
��1������250mL0.1mol/L��������Һ
Ӧ��ȡ�������/mL | Ӧѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ���������� |
_______ | _______ | ______________ |
��2������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�_____________��
A����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
B������Ͳȷ��ȡ�����Ũ�����������ز����������ձ��У��ټ�������ˮ��Լ30mL�����ò���������������ʹ���Ͼ���
C��������ȴ�������ز�����ע��250mL������ƿ��
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�����
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
��3������A�У���ϴ��Һ����������ƿ����Ŀ����________________________����Һע������ƿǰ��ָ������£�������Ϊ_________________________________��
��4�������������������������ҺŨ�Ƚ��к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족��?��û�н���A����_______________����������ˮʱ���������˿̶���_______________��������ʱ���ӿ̶���___________________��δ��ȴ����Һ__________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��![]() ��
��![]() �������ƣ�
�������ƣ� ![]() ��ˮ��Ӧ���������֣�д�����ܵķ���ʽ��
��ˮ��Ӧ���������֣�д�����ܵķ���ʽ��
��___________________________
��___________________________
��Ҫ��ʽ�����ʱ����ýṹʽ��ʾ������H2+Cl2==2HCl�ɱ�ʾΪH��H+Cl��Cl ==2H��Cl������ʵ����ʵ�ж�����һ�ֿ���?
___________________(��ʾ��AgI�ǻ�ɫ����)�� ![]() ��Cl ��________�ۣ����������______________��HIO�ĵ���ʽ________________��
��Cl ��________�ۣ����������______________��HIO�ĵ���ʽ________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з���������ȷ���ǣ�
�����ȼ���������Ȫˮ����ˮ������Ϊ����� ��NaOH��HD��IBr��Ϊ������
���������ռ�����Ϊǿ����� ��C60�����ʯ��ʯī��Ϊ̼��ͬ��������
����ơ�������Һ��ˮ�������ײ��Ͼ�Ϊ����
A. �٢ڢۢ� B. �ۢܢ� C. �ڢܢ� D. �ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����pH��ֽ�ⶨij��ɫ��Һ��pHʱ���淶�IJ����ǣ� ��
A����pH��ֽ������Һ�й۲�����ɫ�仯��������ɫ���Ƚ�
B������Һ����pH��ֽ�ϣ�������ɫ���Ƚ�
C���ø���Ľྻ������պȡ��Һ������pH��ֽ�ϣ�������ɫ���Ƚ�
D�����Թ��ڷ���������Һ����У���pH��ֽ���ڹܿڣ��۲���ɫ��������ɫ���Ƚ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����Ҫ����д�������⣺
��1��ijЩ���ۻ������H2O��NH3��N2O4�ȣ���Һ̬ʱ�ᷢ�����ĵ��룬�磺2H2O ![]() H3O+ + OH��,��Һ̬NH3����ķ���ʽ��
H3O+ + OH��,��Һ̬NH3����ķ���ʽ��
��2��ij�¶ȣ�t�棩ʱ�����0.01mol/L��NaOH��Һ��pH=10������¶���ˮ��KW= ���ڴ��¶��£���pH=b��NaOH��ҺVb L��pH=a��H2SO4��ҺVa L��ϣ������û��ҺΪ���ԣ���a+b=13����Va��Vb=
��3��25��ʱ��0.1mol/L��HA��Һ��![]() =1010����ش��������⣺
=1010����ش��������⣺
��HA�� ���ǿ����ʡ���������ʡ�����
���ڼ�ˮϡ��HA��Һ�Ĺ����У�����ˮ�������Ӷ�������� ������ĸ��
A��c(HA) B��![]() C��c(H+)��c(OH-)�ij˻� D��c(OH-)
C��c(H+)��c(OH-)�ij˻� D��c(OH-)
��4���ڳ����£��к���ͬ�������ͬpH��Ba(OH)2��NH3H2O��NaOH����ϡ��Һ������ͬŨ�ȵ����������ֱ�ΪV1��V2��V3�������ֹ�ϵΪ ���á�V1��V2��V3���͡���������=����ʾ����
��5������A������B������C���� �����
����ͬ�����ͬŨ�ȵ��������У��ֱ����������̼�����Ʒ�ĩ������ͬ�����²���CO2������ɴ�С��˳���� ������д�����Ų��÷��š���������=�����ӣ���ͬ��
����ͬ�����ͬpH���������У�ͬʱ������״���ܶȡ�������ȫ��ͬ��п������������ͬ�����������ͬ��ͬѹ�£�����ʼ��Ӧʱ���ʴ�С��ϵ�� ����Ӧ����ʱ�䳤�̹�ϵ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ס��ҡ����������ֶ�����Ԫ�ص�λ����ͼ��ʾ(�����ҡ���������λ��δ���)��

��֪����Ԫ�ص�ԭ������֮��Ϊ36���ҵ�ԭ��������ס���ԭ������֮����ȡ�
(1)��Ԫ�������ڱ��е�λ��Ϊ______________��
(2)���������γ�AB2�ͻ������ṹʽΪ________��
(3)������Ȼ�����γɻ�������������Ԫ�أ��䵥�����Ԫ�ص�����������Ӧˮ�����Ũ��Һ����ʱ��Ӧ�Ļ�ѧ����ʽ��______________________��
(4)���ס��ҡ�����������Ԫ����������ʱ��������������Ԫ�صõ��Ļ�����������࣬д����Щ���������������ַ������������Ļ�ѧʽ_______________��________________________��(��ԭ�Ӹ�����Ϊ1��2���ڷ����м۵�������Ϊż��)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������й�ͼ��˵����ȷ���ǣ� ��
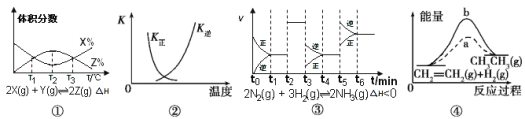
A����ͼ��֪����Ӧ��T1��T3���ﵽƽ�⣬�Ҹ÷�Ӧ�ġ�H��0
B��ͼ�������߱�ʾ��Ӧ2SO2(g)+O2(g) ![]() 2SO3(g),��H<0�����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
2SO3(g),��H<0�����淴Ӧ��ƽ�ⳣ��K���¶ȵı仯
C����ͼ��֪����Ӧ��t6ʱ��NH3����������t3ʱ��ȡ���ͷ�Ӧ�¶ȵĴ�ʩ
D��ͼ����a��b���߷ֱ��ʾ��ӦCH2= CH2(g)+H2(g) ![]() CH3CH3(g) ��H<0ʹ�ú�δʹ�ô���ʱ, ��Ӧ�����е������仯
CH3CH3(g) ��H<0ʹ�ú�δʹ�ô���ʱ, ��Ӧ�����е������仯
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com