


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 3NH3��g����H=-92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶã��ҹ��ϳɰ���ҵĿǰ����������Ϊ������-����ý���¶�-400��500�棬ѹǿ-30��50MPa��
3NH3��g����H=-92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶã��ҹ��ϳɰ���ҵĿǰ����������Ϊ������-����ý���¶�-400��500�棬ѹǿ-30��50MPa��| 4xV2 |
| 27n2(1-x)4 |
| 4xV2 |
| 27n2(1-x)4 |
| �� �� | ȼ���ȣ�kJ?mol-1�� |
| H2��g�� | -285.8 |
| CO��g�� | -283.0 |
| CH4��g�� | -890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ���� | ��H��kJ/mol�� |
| H2��g�� | -285.8 |
| CO��g�� | -283.0 |
| CH4��g�� | -890.3 |
| 4n |
| 4n-2na |
| 4n |
| 4n-2na |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 2������NH3��g����H=-92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶã�
2������NH3��g����H=-92.2kJ/mol���ϳɰ���ҵ��ԭ����N2�ɴӿ����з���õ���H2���ü����ڸ�������ˮ������Ӧ�Ƶã�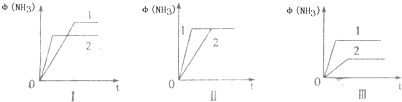
 H2��g��+CO��g����H=+131.3kJ����S=+133.7J/K�÷�Ӧ�ڵ������ܷ��Է�
H2��g��+CO��g����H=+131.3kJ����S=+133.7J/K�÷�Ӧ�ڵ������ܷ��Է�| �� �� | ȼ���ȣ�kJ?mol-1�� |
| H2��g�� | -285.8 |
| CO��g�� | -283.0 |
| CH4��g�� | -890.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com