
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |
| c(��)��V(��) |
| V(����) |


 �߽�������ϵ�д�
�߽�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ����һ�и���5��ģ�⿼�������ۺϻ�ѧ�Ծ����������� ���ͣ������
��15�֣�ij�о�С���һԪ�л�����HA���ܼ�����ˮ�Ļ����ϵ���ܽ�̶Ƚ����о�����25��ʱ������HA��ˮ�в��ֵ��룬HA�ĵ����Ϊ0.10���ڱ��в��ַ���˫�ۣ�����(HA)2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K=C��HA��B/C��HA��W=1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1����֪��lg2=0.3��lg3=0.5 ������Ϣ���£�
| 25��ƽ����ϵ������ˮ��HA�� | ƽ�ⳣ�� | �ʱ� | ��ʼ��Ũ�� |
| ��ˮ�У�HA === H����A�� | K1 | ��H1 | 3.0��10��3 mol��L��1 |
| �ڱ��У�2HA == (HA)2 | K2 | ��H2 | 4.0��10��3 mol��L��1 |
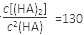 ����Ӧ��_______������С�
����Ӧ��_______������С� (HA)2����֪�ö��۷�Ӧ�ķ�Ӧ����ֵԼΪ��ܵ�5/9 ������������ϵͼ��������� ��
(HA)2����֪�ö��۷�Ӧ�ķ�Ӧ����ֵԼΪ��ܵ�5/9 ������������ϵͼ��������� ��
 ��HA��2��ƽ�ⳣ��K2�����
��HA��2��ƽ�ⳣ��K2�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ����5��ģ�⿼�������ۺϻ�ѧ�Ծ��������棩 ���ͣ������
��15�֣�ij�о�С���һԪ�л�����HA���ܼ�����ˮ�Ļ����ϵ���ܽ�̶Ƚ����о�����25��ʱ������HA��ˮ�в��ֵ��룬HA�ĵ����Ϊ0.10���ڱ��в��ַ���˫�ۣ�����(HA)2����ƽ����ϵ�У�һԪ�л�����HA���ܼ�����B����ˮ��W���еķ���ϵ��ΪK��K=C��HA��B/C��HA��W=1.0�����ﵽƽ����Է�����ʽ���ڵ�HA�ڱ���ˮ�����ܼ��еı���Ϊ1��1����֪��lg2=0.3��lg3=0.5 ������Ϣ���£�
|
25��ƽ����ϵ������ˮ��HA�� |
ƽ�ⳣ�� |
�ʱ� |
��ʼ��Ũ�� |
|
��ˮ�У�HA === H����A�� |
K1 |
��H1 |
3.0��10��3 mol��L��1 |
|
�ڱ��У�2HA == (HA)2 |
K2 |
��H2 |
4.0��10��3 mol��L��1 |
�ش��������⣺
��1������25��ʱˮ��Һ��HA�ĵ���ƽ�ⳣ��K1= ��
��2��25�棬��ˮ��Һ��pHΪ ���ڱ���ϵ��HA��ת����Ϊ__________��
��3��25������ϵ�У�HA�ڱ��з������ۣ������ijʱ����Һ����Ũ������ ����Ӧ��_______������С�
����Ӧ��_______������С�
��4���ڱ��У�HA�Է����з������ۣ�2HA (HA)2�� ��֪�ö��۷�Ӧ�ķ�Ӧ����ֵԼΪ��ܵ�5/9 ������������ϵͼ���������
��
(HA)2�� ��֪�ö��۷�Ӧ�ķ�Ӧ����ֵԼΪ��ܵ�5/9 ������������ϵͼ���������
��

��5�������йظû����ϵ˵������ȷ���� ��
A���÷�Һ©������õ�ˮ��Һ�ͱ���Һ����ˮ��Һ�м�������ˮ������Һ�м���������������ƽ������ƣ���c(HA)����С��
B�������¶ȣ�HA�ĵ���ƽ�ⳣ��K1��2HA  ��HA��2��ƽ�ⳣ��K2�����
��HA��2��ƽ�ⳣ��K2�����
C����������к͵ζ��ķ������������Բ��HA����ʼ��Ũ�ȡ�
D����25��ʱ�����ټ���һ������HA���壬��ˮ�ͱ���HA����ʼ��Ũ��֮����Ϊ3:4��
��6����25��ʱ����0.1000mol/L����������Һ�ζ�20.00mL 0.1000mol/L HAˮ��Һ��������ͼ�л����ζ�����ʾ��ͼ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Ͳ��ȡŨ��������0.01 mol��L-1ϡ����ʱ����Ͳ������ˮϴ����δ������ֱ����ȡŨ���� ������ϡ���ᶨ��ʱ����������ƿ�̶��� �۵ζ�����ʱ���������ֵζ����¶˼��촦������һ��Һ�� �ܵζ�����������������ˮ����ƿ�ڱ�ճ�����������
A.���٢� B.���ڢ� C.���ڢۢ� D.�٢ڢۢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com