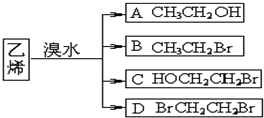
��������C��Ũ���ᡢ��������������E����֪CΪHOCH
2-CH
2OH����ϩ����ˮ��Ӧ����A��A����������ˮ��Һ������������ˮ�������Ҷ�������AΪBrCH
2-CH
2Br��C��Cu����������O
2��������K��K������������ͭ����M����KΪOHC-CHO��MΪHOOC-COOH���Ҷ������Ҷ�������������Ӧ���ɻ�״������N��N�ķ���ʽΪC
4H
4O
4��NΪ

����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC
2H
5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH
2-CH
2OH��DΪBrCH
2-CHO��FΪBrCH
2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH
2-COONa��GΪHOCH
2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ
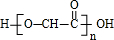
������������Ӧ�ɻ�״������I��IΪ
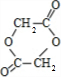
���ݴ˽��
����⣺��C��Ũ���ᡢ��������������E����֪CΪHOCH
2-CH
2OH����ϩ����ˮ��Ӧ����A��A����������ˮ��Һ������������ˮ�������Ҷ�������AΪBrCH
2-CH
2Br��C��Cu����������O
2��������K��K������������ͭ����M����KΪOHC-CHO��MΪHOOC-COOH���Ҷ������Ҷ�������������Ӧ���ɻ�״������N��N�ķ���ʽΪC
4H
4O
4��NΪ

����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC
2H
5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH
2-CH
2OH��DΪBrCH
2-CHO��FΪBrCH
2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH
2-COONa��GΪHOCH
2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ
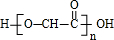
������������Ӧ�ɻ�״������I��IΪ

��
��1��������Cԭ�ӣ�����Hԭ�ӱ���C���ļ۽ṹ����E�Ľṹ��ʽ��֪�������к���8��Cԭ�ӡ�16��Hԭ�ӡ�4��Oԭ�ӣ���E�ķ���ʽ��C
8H
16O
4��
�ʴ�Ϊ��C
8H
16O
4��
��2��������������֪��BΪBrCH
2-CH
2OH��IΪ

��NΪ

��
�ʴ�Ϊ��BrCH
2-CH
2OH��

��

��
��3��G��I��HOCH
2-COOH����������Ӧ���ɻ�״������

��
��������������������Ϊͬ���칹�����N��I��
�ʴ�Ϊ��������Ӧ��N��I��
��4��д������ת���Ļ�ѧ����ʽ��
C��K��HOCH
2-CH
2OH��Cu����������O
2��������OHC-CHO����Ӧ����ʽΪ��
HOCH
2-CH
2OH+O
2OHC-CHO+2H
2O��
F��J��BrCH
2-COOH��������ˮ��Һ����������������HOCH
2-COONa����Ӧ����ʽΪ��
BrCH
2-COOH+2NaOH
HOCH
2-COONa+NaBr+H
2O��
�ʴ�Ϊ��HOCH
2-CH
2OH+O
2OHC-CHO+2H
2O��BrCH
2-COOH+2NaOH
HOCH
2-COONa+NaBr+H
2O��


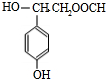
 ���Ľṹ�ɼ�дΪ
���Ľṹ�ɼ�дΪ  ��E�ķ���ʽ��
��E�ķ���ʽ��



 ����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC2H5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH2-CH2OH��DΪBrCH2-CHO��FΪBrCH2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH2-COONa��GΪHOCH2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ
����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC2H5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH2-CH2OH��DΪBrCH2-CHO��FΪBrCH2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH2-COONa��GΪHOCH2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ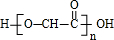 ������������Ӧ�ɻ�״������I��IΪ
������������Ӧ�ɻ�״������I��IΪ ���ݴ˽��
���ݴ˽�� ����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC2H5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH2-CH2OH��DΪBrCH2-CHO��FΪBrCH2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH2-COONa��GΪHOCH2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ
����ϩ����ˮ��Ӧ����B��B�ķ���ʽΪC2H5BrO��B����������D��D��������F����B�к���-OH����BΪBrCH2-CH2OH��DΪBrCH2-CHO��FΪBrCH2-COOH��F����������ˮ��Һ����������������J��J�ữ����G����JΪHOCH2-COONa��GΪHOCH2-COOH��G����������Ӧ���ɸ߷��ӻ�����H��HΪ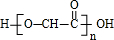 ������������Ӧ�ɻ�״������I��IΪ
������������Ӧ�ɻ�״������I��IΪ ��
�� ��NΪ
��NΪ ��
�� ��
�� ��
�� ��
��

 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�

 ���Ľṹ�ɼ�дΪ
���Ľṹ�ɼ�дΪ ��E�ķ���ʽ��
��E�ķ���ʽ��



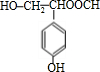


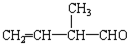 ���������ŵ�������
���������ŵ�������