

| ���� | ʵ����� | ʵ������ | ʵ����� |
| �� | ����a����μ���H2SO3��Һ������ | ��ƿ����Һ�ɳȻ�ɫ��Ϊ��ɫ | Br2�������Դ���H2SO3 |
| �� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | H2O2�������Դ���Br2 |

���� �����������Է���������ԭ��Ӧ���������������Դ������������ԭ�����ʵ�飬̽��H2O2��H2SO3��Br2������ǿ�����漰ʶ������A�����ƣ���Ϸ�Ӧԭ������ʵ������п��ܳ��ֵ����������ӷ���ʽ��ʾ��Ӧԭ��������������ȼҵ��������ԭ����
��1����Ӧ�����Ϸ�����A�������������ݳ����������ɳ�������ʶ���A�����ƣ�
��2�����ݽ���Br2�������Դ���H2SO3��֪����ˮ��ǿ�����ԣ�������H2SO3������H2SO3��Һ��μ��룬��Һ���������С�������Һ����ɫ���dzֱ����ɫ����������õķ�Ӧ��Һ����μ���H2O2��Һ����Һ�ֱ�Ϊ�Ȼ�ɫ���ɼ���Һ���Br-�ֱ�����ΪBr2��˵����H2O2�����Դ���Br2��
��3����Ϊ�����μӵ�H2SO3��Һ�ǹ����ģ���ʼ����H2O2��Һʱû��ֱ��������Һ���Br-���������������H2SO3������������ԭ��Ӧ���ʲ�����е����H2O2�ֱ���H2SO3��Br-�����˷�Ӧ��
��4�����װ��Ϊ��ⱥ��ʳ��ˮ�ĵ��أ��������ȼҵ��������ɵõ��������������ƣ����ӽ���Ĥ��������OH-����Ϊ�����OH-�����ɵ��������������ܽ��ڼ�����Һ��Ƶõ��������Ʋ�����
��� �⣺��1������AΪ�����Ļ�ѧ������������ʶ��ӦΪ���������ܣ��ʴ�Ϊ�����������ܣ�
��2����Ӧ�����еμ�H2SO3��Һ����ˮ�е���ᱻ��ԭΪBr-����ˮΪ�Ȼ�ɫ�����ŵμӵ�H2SO3��Һԭ��Խ�࣬��Һ�����Խ��Խ�٣���ɫ����dz��������ɫ���������������H2O2��Һ���ʸտ�ʼ��Һ��ɫû�б仯�������μ���Һ��Ϊ�Ȼ�ɫ��˵��H2O2��������Һ���Br-����Br2��������Ӧ�Ļ�ѧ����ʽΪ2H2O2+2HBr=Br2+2H2O������������ԭ��Ӧ��֪H2O2�������Դ���Br2��
�ʴ�Ϊ���ɳȻ�ɫ��Ϊ��ɫ��H2O2�������Դ���Br2��
��3���������������H2O2��Һ������H2SO3����H2SO3��ȫ��������������Һ���Br-��������Һ���ɫ�����ձ�Ȼ�ɫ�������ķ�Ӧ���ӷ���ʽΪH2SO3+H2O2=2H++SO42-+H2O��2H++2Br-+H2O2=Br2+2H2O��
�ʴ�Ϊ������1��H2SO3�й�����H2O2�Ⱥ�H2SO3��Ӧ��H2SO3+H2O2=2H++SO42-+H2O��2H++2Br-+H2O2=Br2+2H2O��
��4���ȼҵ�õ��IJ������������������������ƣ����OH-������Ĥ���������������ɵ��������������ܽ��ڼ�����Һ��õ����������Ʋ�������Ӱ�������
�ʴ�Ϊ��NaOH��H2������Cl2��NaOH��Ӧ����NaClO��Ӱ��NaOH�IJ����ʹ��ȣ�
���� ������������ԭ��Ӧ���������������Դ�����������Ϊ���壬̽��H2O2��H2SO3��Br2������ǿ����������ʵ��Ļ���������������ѧ�����ʵ���������ԭ������������һ�����Ѷȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����������һ���ǽ��������� | |
| B�� | ����������һ���Ǽ��������� | |
| C�� | �������������������һ��������ˮ��Ӧ������Ӧ���ᡢ�� | |
| D�� | ����������һ���Ƿǽ��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ͨп�̸ɵ��̼���Ǹ�����пƬ������ | |
| B�� | ʢˮ���������ڿ�����ˮ���紦��������ʴ | |
| C�� | Ϊ��ֹ�����ĸ�ʴ���ڽ�������Ϳ���ᡢ��֬ | |
| D�� | ��������п��Ӧ��ȡ�������������ʵ�п�ȴ�п���������ٶȿ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �������� | ��ȫ����pH |
| Fe2+ | 9.7 |
| Mg2+ | 12.4 |
| Fe3+ | 3.2 |
| Al3+ | 5.2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��֪����CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H20��CH2=CH2+Br2��BrCH2-CH2Br
��֪����CH3CH2OH$��_{170��}^{ŨH_{2}SO_{4}}$CH2=CH2��+H20��CH2=CH2+Br2��BrCH2-CH2Br| �Ҵ� | 1��2-�������� | ���� | |
| ͨ��״���µ�״̬ | ��ɫҺ�� | ��ɫҺ�� | ��ɫҺ�� |
| �ܶ�/g•cm-3 | 0.79 | 2.2 | 0.71 |
| �۵�/�� | -130 | 9 | -116 |
| �е�/�� | 78.5 | 132 | 34.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
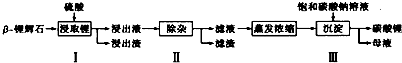
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| 2min | 4min | 6min | 8min | �� | |
| CO | 0.07 | 0.06 | 0.06 | 0.05 | �� |
| H2 | x | 0.12 | 0.12 | 0.2 | �� |
| CH3OH | 0.03 | 0.04 | 0.04 | 0.05 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 25��ʱ��������ĵ��볣��Ϊ��
25��ʱ��������ĵ��볣��Ϊ��| ��ѧʽ | CH3COOH | H2CO3 | HClO |
| ����ƽ�ⳣ��K | 1.8��10-5 | K1=4.3��10-7 K2=5.6��10-11 | 3.0��10-8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ���� | ���ͻ���� |
| A | ij��Һ��CCl4�������� | �²���Һ����ɫ | ԭ��Һ��һ����I2 |
| B | ����Ʒ��Һ���ȵμӹ�����ϡ���ᣬ�ٵμ�BaCl2��Һ | �μ�ϡ���������μ�BaCl2����ְ�ɫ������ | ˵����Ʒ��Һ��һ������SO42- |
| C | ��ij��Һ�м������������ټ���ϡ���� | �а�ɫ���� | ԭ��Һ����Cl- |
| D | ��ij��Һ�м���ϡ���� | ������ɫ���� | ˵��ԭ��Һ��һ����CO32- |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com