
������أ�K2FeO4����һ�ּ�������������������һ������Ͷ��ˮ���������������������£�
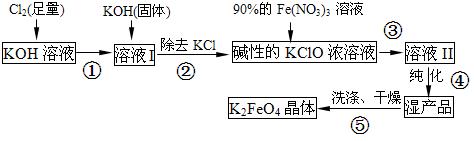
��1������KOH��Һʱ������ÿ100 mLˮ���ܽ�61.6 g KOH���壨����Һ���ܶ�Ϊ1.47 g/mL�����������ʵ���Ũ����������____mol/L��
��2������ҺI�м���KOH�����Ŀ���� �����ţ���
A������ҺI�й�����Cl2������Ӧ�����ɸ����KClO
B��KOH�����ܽ�ʱ��ų��϶����������������߷�Ӧ����
C��Ϊ��һ����Ӧ�ṩ���ԵĻ���
D��ʹ������KClO3ת��Ϊ KClO
��3������ҺII�з����K2FeO4���õ�����ƷKNO3��KCl��д�����з�Ӧ�����ӷ���ʽ��
��
��4��������أ�K2FeO4����ˮ��Ӧʱ�����ɺ��ɫ�����ͬʱ�ͷų�һ�־��������Ե����嵥�ʣ���д���÷�Ӧ�����ӷ���ʽ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л��������ұ�(GB2760��200��)�涨���Ѿ���SO2���ʹ����Ϊ0.25g��L��1.ij��ȤС������9ͼIװ��(�г�װ����)�ռ�ij���Ѿ���SO2,�����京�����вⶨ.
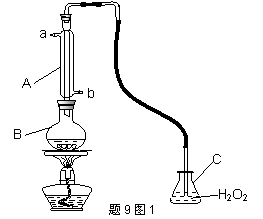

 ��1������A�������� ��ˮͨ��A�Ľ���Ϊ ��
��1������A�������� ��ˮͨ��A�Ľ���Ϊ ��
��2��B�м���300.00mL���Ѿƺ��������ᣬ����ʹSO2ȫ���ݳ�����C��H2O2��ȫ��Ӧ���仯ѧ����ʽΪ ��
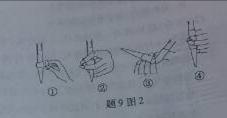
��3����ȥC�й�����H2O2��Ȼ����0.0900mol��L��1NaOH����Һ���еζ����ζ�ǰ������ʱ��Ӧѡ����9ͼ2�е� �����ζ��յ�ʱ��Һ��pH��8.8����ѡ���ָʾ��Ϊ ������50mL�ζ��ܽ���ʵ�飬���ζ����е�Һ���ڿ̶ȡ�10�����������Һ������ (�����)
(�٣�10mL���ڣ�40mL���ۣ�10mL���ܣ�40mL)
��4���ζ����յ�ʱ������NaOH��Һ25.00mL�������Ѿ���SO2����Ϊ g��L��1
��5���òⶨ�����ʵ��ֵƫ�ߣ�����ԭ����������װ������Ľ���ʩ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������A(����ʽΪC6H6O)��һ���л�����ԭ�ϣ��ڿ������ױ�������A���й�ת����Ӧ���� (���ַ�Ӧ������ȥ)��
(���ַ�Ӧ������ȥ)��
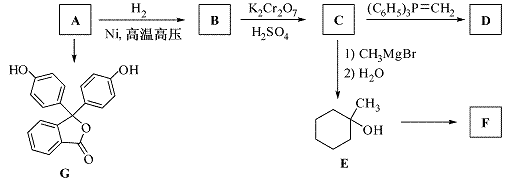
��֪����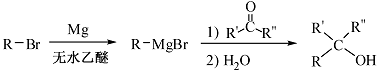
��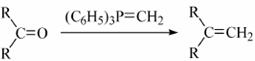 (R��ʾ������R���R���ʾ��������)
(R��ʾ������R���R���ʾ��������)
(1)д��A�Ľṹ��ʽ��  ��
��
(2)G�dz���ָʾ����̪��д��G�к��������ŵ����ƣ� �� ��
(3)ij��������E��ͬ���칹�壬�ҷ�����ֻ�����ֲ�ͬ��ѧ�������⡣д���û�����Ľṹ��ʽ�� (��дһ��)��
(4)F��D��Ϊͬ���칹�塣д����ӦE��F�Ļ�ѧ����ʽ��
��
(5)��������֪ʶ����������Ϣ��д����A��HCHOΪԭ���Ʊ�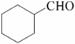 �ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ(���Լ�����)���ϳ�·������ͼʾ�����£�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ˮ����������Һ��������ʵ��ǣ�������
A��AgNO3 B��Na2CO3 C��NaCl D��KI
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������зḻ��ʳƷ���������Դ��ҩ���ˮ����Դ��(��ͼ��ʾ)�������й�˵���������( ��)
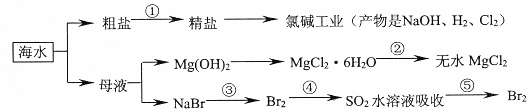
A��������ת���ĽǶ��������ȼҵ�еĵ�ⱥ��ʳ��ˮ��һ��������ת��Ϊ��ѧ�ܵĹ���
B�����̢��нᾧ����MgCl2��6H2OҪ��HCl�ķ�Χ�м�����ˮ�Ƶ���ˮMgCl2
C���ڹ��̢ۡ�������Ԫ�ؾ�������
D����ȥ�����е�SO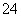 ��Ca2����Mg2����Fe3�������ʣ������ҩƷ˳��ΪNa2CO3��Һ��NaOH��Һ��BaCl2��Һ�����˺������
��Ca2����Mg2����Fe3�������ʣ������ҩƷ˳��ΪNa2CO3��Һ��NaOH��Һ��BaCl2��Һ�����˺������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ڽ������Һ������������������ȷ����
A����Һ�ʵ����ԣ�������е��
B����Һ��������һ�������磬�����з�ɢ�������е��
C����Һ�з�ɢ����������ֽ�������з�ɢ������������ֽ
D����Һ��ͨ��һ������û��������������ͨ��һ�����߳��������Ĺ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и���������ָ������Һ���ܴ����������
����ɫ��Һ�У�K����Cu2����Na����SO42- ��pH��11����Һ�У�CO32- ��Na����AlO2����NO3��
�ۼ���Al�ܷų�H2����Һ�У�Cl����HCO3����NO3����NH4��
������ˮ�������c��OH������10��13 mol��L��1����Һ�У�Na����Ba2����Cl����I��
����ʹ��ɫʯ����ֽ��Ϊ��ɫ����Һ��Na����Cl����S2����ClO��
��������Һ�У�Fe2����Al3����NO3����Cl��
A���٢ڢ� B���ۢݢ� C���ڢ� D���ڢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij����������ܺ�Al����NH4��2SO4��MgCl2��A1Cl3��FeCl2��NaCl�е�һ�ֻ��֣��ֶԸû����������ʵ�飬����������й�������ͼ����������ѻ���ɱ�����������
ij����������ܺ�Al����NH4��2SO4��MgCl2��A1Cl3��FeCl2��NaCl�е�һ�ֻ��֣��ֶԸû����������ʵ�飬����������й�������ͼ����������ѻ���ɱ�����������
�ش��������⣺
��1����������Ƿ����FeCl2 ����ǡ�����
��2����������Ƿ����(NH4)2SO4 ����ǡ���������ж������� ��
��3��д����Ӧ�ܵ����ӷ�Ӧʽ�� ��
��4������ݼ������жϻ�������Ƿ���AlCl3 ����ǡ���,����ж������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��NAΪ����٤��������ֵ������˵���������
A��1 mol 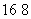 OD�� ���Ӻ��е����ӡ���������Ϊ9NA
OD�� ���Ӻ��е����ӡ���������Ϊ9NA
B��3.6 gʯī��C60�Ļ�����У����е�̼ԭ����Ϊ0.3NA
C������4.6 g��Ԫ�صĹ������ƺ������ƵĻ�����У�������������Ϊ0.3NA
D����״���£�4.48 L���麬�еķ�����Ϊ0.2NA
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com