
| 5��112(W2-W1) |
| 160a |
| 5��112(W2-W1) |
| 160a |
| 1 |
| 5 |
| 112 |
| 160 |
| 5��112(W2-W1) |
| 160a |
| 5��112(W2-W1) |
| 160a |


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| 7(W2-W1) |
| a |
| 7(W2-W1) |
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

10(W2-W1)��
| ||
| a |
10(W2-W1)��
| ||
| a |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�����и���5�·�ģ�⿼�Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
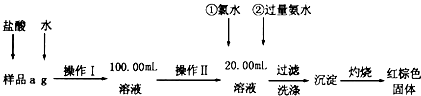
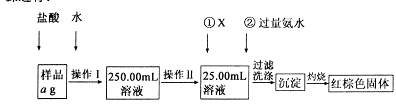
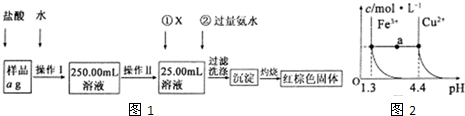
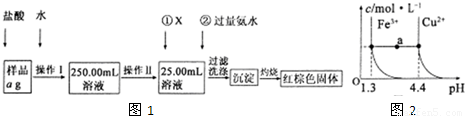
ij�Ȼ�����Ʒ������FeC12���ʡ���Ҫ�ⶨ������Ԫ�ص�����������ʵ�鰴���²�����У�
�������ͼ���̣��ش��������⣺
��1�����������õ��IJ����������ձ����������⣬�������� �� �����������ƣ�������������õ��������� �����ţ���
A��50mL�ձ� B��50mL��Ͳ C��100mL��Ͳ D��25mL��ʽ�ζ���
��2���ٵ�X��ѡ�ã��ѧʽ������Ŀ���� �����백ˮҪ������ԭ���� ��
��3����������Ƿ��Ѿ�ϴ�Ӹɾ��IJ����� ��
��4����������������W1g������������Ⱥ����������W2g������Ʒ����Ԫ�ص����������� ���г�����ʽ�����軯�����������ʱδ��ַ�Ӧ�������ղ����Ľ�� ����ƫ����ƫС���������䡱����
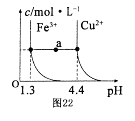
��5����֪�ڳ�����Cu2+��Fe3+����Һ����pH�仯ʱˮ���������ͼ��ʾ
 ��
��
��ͼ��a���˵����ȷ���� ������ţ���
�ټ�����NH4C1�����ʹ��Һ��a����ˮƽ����䵽Cu2+�����ϡ�
����Һ��a���ˮƽ����Fe3+��Cu2+����������ص��C(H+)��c(OH��)�˻����
��Fe(OH)3��Cu(OH)2��a���������Һ�о��ﵽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�������ģ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�����и߿���ѧ��ģ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com