
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ����_________________��
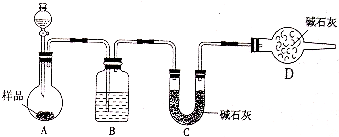
��2��������������ͼװ�ý���ʵ�顣���ش��������⣺
�ٷ�Һ©����Ӧ��װ___________������ᡱ��ϡ���ᡱ����Dװ�õ�������_________________________________��
��ʵ���г�������Ʒ�����⣬�����________װ�ã�����ĸ��ʾ��ǰ�������ı仯��
��3������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������_____________��
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g�������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ____________������һλС������
����12�֣�
��1��ʹNaHCO3�ֽ���ȫ
��2����ϡ���ᣬ��ֹ���CO2��H2O��װ��C�еļ�ʯ�����ա� ��C������ĸ��ʾ����
��3���ٲ���������55.8%������һλС��������ÿ��2�֣�
���������������1��С�մ�NaHCO3�������ֽ⣬�ʼ��������ص�Ŀ����ʹNaHCO3�ֽ���ȫ��
��2���������ӷ����ʷ�Һ©����Ӧ��װϡ���ᣬ��ʯ�������տ����е�ˮ������CO2����Dװ�õ������Ƿ�ֹ���CO2��H2O��װ��C�еļ�ʯ�����ա�
��A����CO2���徭B�����C�н������գ��ʻ����Cװ��ǰ�������ı仯��
��3���ٹ��˳��õIJ����������ձ���©���Ͳ�������
�ڷ�����ӦNa2CO3+Ba��OH��2=BaCO3��+2NaOH��NaHCO3+Ba��OH��2=BaCO3��+NaOH+H2O��
��Ʒ9.5g������ij���̼�ᱵ����Ϊ19.7g�����ʵ���Ϊ19.7g/(197g/mol) =0.1mol������Ʒ��̼���Ƶ����ʵ���Ϊxmol��̼�����Ƶ����ʵ���Ϊymol����106x+84y=9.5��x+y=0.1�����x=0.05��y=0.05������̼���Ƶ�����Ϊ106g/mol��0.05mol=5.3g������̼������������Ϊ5.3g/9.5g��100%=55.8%��
���㣺̽�����ʵ���ɻ�������ʵĺ��� �Ƶ���Ҫ������
��������ʵ��̽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������Ϊ���壬����ѧ������ʵ��ԭ����װ���������ۡ�ʵ�������������ѧ����ȣ��Ѷ��еȣ���Ŀ�漰����С�մ�ʹ���Ļ�ѧ֪ʶ�Ƕ��ģ�������һ����Ƕȵ�̽���⡣



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д��������������
ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ���ü��õ�С�մ���Ʒ�д���������������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��������������е�����ѧ������ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��������������и�����ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
��14�֣�ijУ��ѧ�о���ѧϰС���������ʵ�鷽�����ⶨ�����Ѿõ�С�մ���Ʒ�д��������������
��1������һ����ȡһ����������Ʒ�����������м��������غ���ȴ������ʣ��������������㡣ʵ���м��������ص�Ŀ���� ��
��2������������ȡһ������Ʒ������С�ձ��У�������ˮ�ܽ⣬��С�ձ��м��������Ȼ�����Һ������ϴ�ӣ���������������������������㣺
�ٹ��˲����У������ձ���©����õ��IJ���������______________________��
���������жϳ����Ƿ���ȫ�ķ�����_______________________________________
���������Լ���Ϊ������������֪�Ƶ���Ʒ9.5g������ij�������Ϊ19.7g������Ʒ��̼���Ƶ���������Ϊ_________________������һλС������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com