
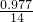 ��
��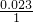 =3��1���������ʽΪHN3��
=3��1���������ʽΪHN3�� =0.1mol�����ݷ�Ӧ2HN3�TH2+3N2����������������ʵ���Ϊ0.1mol��2=0.2mol�����Ϊ0.2mol��22.4L/mol=4.48L��
=0.1mol�����ݷ�Ӧ2HN3�TH2+3N2����������������ʵ���Ϊ0.1mol��2=0.2mol�����Ϊ0.2mol��22.4L/mol=4.48L��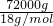 =4000mol�������ĵ�n��N2H4��=2000mol������������Ϊ2000mol��32g/mol=64000g����64Kg��
=4000mol�������ĵ�n��N2H4��=2000mol������������Ϊ2000mol��32g/mol=64000g����64Kg��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2013?����һģ�����۾ź��롰�칬һ�š��ɹ��Խӣ����ҹ����˺�����ҵ��չ��������һ����־���ռ�ʵ���ҡ��칬һ�š��Ĺ���ϵͳΪ��������ȼ�ϵ�أ�RFC����RFC��һ�ֽ�ˮ��⼼��������ȼ�ϵ�ؼ������ϵĿɳ���أ�ͼΪRFC����ԭ��ʾ��ͼ�������й�˵����ȷ���ǣ�������
��2013?����һģ�����۾ź��롰�칬һ�š��ɹ��Խӣ����ҹ����˺�����ҵ��չ��������һ����־���ռ�ʵ���ҡ��칬һ�š��Ĺ���ϵͳΪ��������ȼ�ϵ�أ�RFC����RFC��һ�ֽ�ˮ��⼼��������ȼ�ϵ�ؼ������ϵĿɳ���أ�ͼΪRFC����ԭ��ʾ��ͼ�������й�˵����ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com