���𰸡�
��������������֪��F�ĵ�����D
2��ȼ�յIJ����ʹƷ����Һ��ɫ����ʹƷ����Һ��ɫ�Ļ������Ƕ�����������F����D������������E�ĵ�����������Ӧ������E
2D��E
2D
2���ֹ��壬E�ڻ������еĻ��ϼ���+1�ۣ�E
2D
2����Ԫ�صĻ��ϼ���-1�ۣ�����E���ƣ�A��Eͬ���壬��A��ԭ��������С��CA
4++DA
-=CA
3��+A
2D�����ַ�Ӧ��������ĵ���������E
+��ȣ�����A����Ԫ�أ�B�ĵ�����������ȼ�տ�����BD��BD
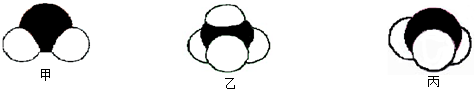
2�������壬A��B��C��D��E��Fԭ������������������B��̼Ԫ�أ�C�ǵ�Ԫ�أ�����A��B��C��D��E��F�ֱ�����Ԫ�ء�̼Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�ء���Ԫ�أ�
��1�����ݶ�����̼�Ľṹд���ṹʽ�����������ӵĺ������д�������ӽṹʾ��ͼ�����ݰ������ӵ�VSEPRģ���ж���ռ乹�ͣ�
��2����������ͭ��ϡ���ᷴӦ��������д��˫��ˮ��ͭ�����ᷴӦ�ķ���ʽ��
��3�����ݷ�Ӧ��������P�ʱ�֮��Ĺ�ϵд���Ȼ�ѧ��Ӧ����ʽ��
��4�����жϸ����ӻ�����Ļ�ѧʽ���ٸ����仯ѧʽд������ʽ��
�辧���ı߳�Ϊacm������
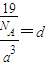
��a=
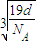
����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ
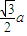
=
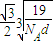
��
����⣺��1��������̼��̼Ԫ�غ���Ԫ���γɵ��ǹ��ۼ�����ֱ���ͽṹ��̼��һ����ԭ��֮��������Թ��õ��Ӷԣ�������ṹʽΪO=C=O���������к���18�����ӣ����������ӽṹʾ��ͼΪ
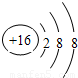
�������������ͽṹ��
�ʴ�Ϊ��O=C=O��
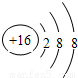
�������ͣ�
��2������ͭ��ϡ���ᷴӦ��������������ͭ��ˮ��˫��ˮ��ǿ�����ԣ�ͭ�л�ԭ�ԣ������������£�˫��ˮ��ͭ�ܷ���������ԭ��Ӧ������˫��ˮ��ͭ��ϡ����ķ�Ӧ����ʽΪ��
Cu+H
2O
2+H
2SO
4=CuSO
4+2H
2O���ʴ�Ϊ��Cu+H
2O
2+H
2SO
4=CuSO
4+2H
2O��
��3��0.5molN
2H
4�μӷ�Ӧ�ų�320KJ����������1molN
2H
4�μӷ�Ӧ�ų�640kJ���������Ը��Ȼ�ѧ��Ӧ����ʽΪ��
N
2H
4��1��+2H
2O
2��1��=N
2��g��+4H
2O��g������H=-640kJ?mol
-1��
�ʴ�Ϊ��N
2H
4��1��+2H
2O
2��1��=N
2��g��+4H
2O��g������H=-640kJ?mol
-1��
��4�����ӻ�����CA
5ΪNH
4H������������Ϊ�����ӣ�������Ϊ笠����ӣ������ʽΪ
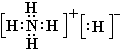
��
�����к��е������Ӹ���Ϊ1�������Ӹ���Ϊ8×

=1���������к���һ����NH
4H���ӡ����辧���ı߳�Ϊacm������
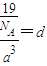
��a=
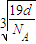
����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ
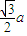
=
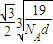
��
�ʴ�Ϊ��
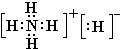
��
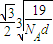
��
���������⿼���Ϊ�ۺϣ��漰ԭ�ӽṹ��Ԫ��������֪ʶ�Լ������ļ����֪ʶ����Ŀ�ѶȽϴ�ע�����þ�̯���жϾ������⣮

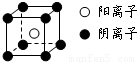
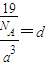 ��a=
��a=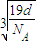 ����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ
����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ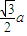 =
=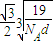 ��
�� �������������ͽṹ��
�������������ͽṹ�� �������ͣ�
�������ͣ�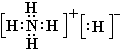 ��
�� =1���������к���һ����NH4H���ӡ����辧���ı߳�Ϊacm������
=1���������к���һ����NH4H���ӡ����辧���ı߳�Ϊacm������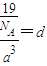 ��a=
��a=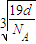 ����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ
����������������������֮�����̾���Ϊ��������ĵľ��룬Ϊ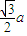 =
=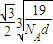 ��
��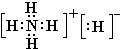 ��
��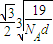 ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�