
��15�֣�ij�о�С���û�ͭ����Ҫ�ɷ���CuFeS2������SΪ-2�ۣ�Ϊ��Ҫԭ����ͭ�����ܷ�ӦΪ��2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2����ʵ�ϸ÷�Ӧ�ǰ��������̷ֲ����еģ�

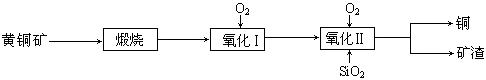
��1��������ķ�Ӧ��Ҫ���������ɵ�����������һ������Ϊ��������������������跴Ӧ���ɿ�������������Ҫ�ɷ��� ���ѧʽ����
��2���ݱ�������һ��ϸ�������������¿��Խ���ͭ�������������Σ���Ӧ����������Һ�з����ġ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3���ҹ�ѧ���о����֣��Ծ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ����������ȴ���ܽ⡢�������ᾧ���õ�CuSO4��5H2O�������ɱ��ܹ��������ࡣ�й�ʵ�������±���
| ����¯�¶�/�� | 560 | 580 | 600 | 620 | 640 | 660 |
| ˮ����Cu/% | 90.12 | 91.24 | 93.50 | 92.38 | 89.96 | 84.23 |
| ������Cu/% | 92.00 | 93.60 | 97.08 | 97.82 | 98.16 | 98.19 |
��15��
��1��FeSiO3��2�֣�
��2��4CuFeS2+2H2SO4+17O2 4CuSO4+2Fe2(SO4)3+2H2O��2�֣�
4CuSO4+2Fe2(SO4)3+2H2O��2�֣�
��3����4CuFeS2+15O2��4CuSO4+2Fe2O3+4SO2��2�֣�
�ڿ��Ƽ���CuFeS2���ٶȣ���CuFeS2��O2��Ӧ���ȣ���2�֣�
��CuSO4���ȷֽ⣨2�֣�
�ܹ��ˣ�2�֣� ����CuO[��Cu(OH)2��CuCO3]��ĩ����ֽ��裬������Һ��pHԼΪ3.2��������У����ˣ��ã�������ϡ�����ữ��3�֣�
���������������1������2CuFeS2+2SiO2+5O2��2Cu+2FeSiO3+4SO2���ɵÿ���ΪFeSiO3��
��2���������֪��CuFeS2��������Һ����������һ��ϸ�������������ɵ�������������ͭ������������ѧ����ʽΪ4CuFeS2+2H2SO4+17O2 4CuSO4+2Fe2(SO4)3+2H2O��
4CuSO4+2Fe2(SO4)3+2H2O��
��3���پ�CuFeS2��Ϊԭ���ڷ���¯����O2����������Ӧ�����յ�CuSO4��5H2O��˵����Ԫ�صĴ�����ʽ������������ˮ�ܽ�ɹ��˳�ȥ������CuFeS2��O2��Ӧ�Ļ�ѧ����ʽΪ4CuFeS2+15O2��4CuSO4+2Fe2O3+4SO2
����ΪCuFeS2��O2��Ӧ���ȣ����������������п��Ƽ���CuFeS2���ٶȣ������¶ȣ�
��ˮ����ͭ������ΪCuSO4?5H2O��������ͭ������ΪCuO���¶Ƚϸ�ʱ��CuSO4?5H2O�ɷֽ�����CuO������600������ʱˮ����ͭ�����ﺬ�����٣�
���������г�����ͭ�����������������������ˮ��������ȴ��ij�����ʵ�����������Ҫ�ǹ��ˣ���ȥ�����Ӷ�����ȥͭ���ӣ�������Һ��pHֵ��3.2��4.7֮�䣬ʹ��������ȫ��������ͭ���Ӳ����������Ծ�������Ǽ���CuO[��Cu(OH)2��CuCO3]��ĩ����ֽ��裬������Һ��pHԼΪ3.2��������У����ˣ��ã�������ϡ�����ữ��
���㣺���������Ʊ�������ͼ�ķ�������ѧ����ʽ����д


 һ����ʦ����Ӧ����������һ��ȫϵ�д�
һ����ʦ����Ӧ����������һ��ȫϵ�д� Сѧѧϰ�ð���ϵ�д�
Сѧѧϰ�ð���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
���ƿ����Ϊ����ʵ��ġ�����ƿ��������Ϊ��������ϲ����ܺ�������������ɸ��ֹ��ܵ�װ�á����и�ͼ����������������ȫƿ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ij��ȤС���ͬѧ��ͬ��������ͼ��ʾ��ʵ��װ�ã��ȿ�������ȡ�����ֿ�������֤���ʵ����ʡ���ش��������⣺
��1������װ�â���ȡ���壬��������ķ�ӦӦ�߱��������� ��
��2������װ�â���ȡ���壨K2�رգ�K1������ͬѧ��Ϊ������װ�â�����ռ�H2��NH3�����壬�������ռ�O2��NO�����壬�������ǣ�
_______________________________________________________________________��
��ͬѧ��Ϊ����װ�â������Ľ������ı�����װ�ã���Ҳ���ռ�O2��NO�����壬�������ռ�NO2���壬�Ľ��ķ����� �������ռ�NO2�����ԭ���ǣ��û�ѧ����ʽ��ʾ�� ��
��3������װ�â� ��֤���ʵ����ʣ�K2��K1�رգ�����Ҫ���ʵ��֤��������
KMnO4��Cl2��Br2�������A �м�Ũ���ᣬB�м� ��C�м� ���۲쵽C�е������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�����к��зḻ�ĵ⡣Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺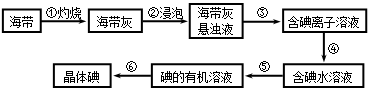
37. ����۵�ʵ�����������_____________�������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ�����������_____________��
38. ������������Լ���________����Ӧ�����ӷ���ʽ��____________________��
39. ������У�ijѧ��ѡ���ñ�����ȡ�⣬������_______________________________��
_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��12�֣�����п���㷺Ӧ���ڹ�ũҵ������ҽҩ����ҵ��������п����Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO�ȣ�����ZnSO4��7H2O��һ���������£�
�Ų������������������������
�����ʱ���費��ͨ�����ˮ������Ŀ���� ��
�ڹ���ʱ��Ϊ��ֹ����������װ���辭��������������Һ��ϴ������ϴԭ����
���û�ѧ����ʽ��ʾ����
�Ʋ�����У���pHԼΪ5.1����Һ�м��������أ�����Fe(OH)3��MnO(OH)2���ֳ������÷�Ӧ�����ӷ���ʽΪ ��
�Dz������������C����Ҫ�ɷ��� ��
��ȡ28.70 g ZnSO4��7H2O��������ͬ�¶ȣ�ʣ�����������仯��ͼ��ʾ��
�ٲ�����еĺ�ɲ������ڼ�ѹ�����½��У���ԭ���� ��
��680 ��ʱ���ù���Ļ�ѧʽΪ ��
a��ZnO b��Zn3O(SO4)2 c��ZnSO4 d��ZnSO4��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����ɫ������A������ɫ���ߵ�ѹ���������ص㣬�����������˵绯ѧ��ĸ߶����ӡ��ڳ��º���������£�������A�����ȶ��Ĵ��ڣ�������ˮ��Һ�в��ȶ���һ��ʱ���ת��Ϊ���ɫ������ͬʱ����һ�����嵥�ʡ�ij��ȤС���ͬѧ�Ի�����A������ɷ�����ȷ��A�н�����O��K��Fe����Ԫ�ء�ȡ3.96g������A�ķ�ĩ����ˮ���μ�������ϡ���ᣬ��Ӧ�����Һ�м��뺬��0.08mol KOH����Һ��ǡ����ȫ��Ӧ�����ˣ���ϴ�Ӻ�ij���������գ��õ�����ɫ�����ĩ1.60g����������Һ��һ�������������ɵõ�һ�ִ����IJ����ᾧˮ����10.44g��
��1��������A�Ļ�ѧʽΪ ��������A��H2O��Ӧ�����ӷ���ʽΪ ��
��2��������A������Ϊһ�֡���ɫ��Ч��ܡ�ˮ��������ԭ���� ��
��3��������A���Ʊ�����ͨ������������д����KOH�����������ô�������������������Ʊ�A�Ļ�ѧ����ʽ ��
��4��Ŀǰ��������Ի�����A���ȶ��Խ����˴�����̽������ȡ����һ���Ľ�չ�������������п����������Aˮ��Һ�ȶ��Ե���
| A���������� | B��KOH | C������ | D��Fe(NO3)3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(15��)����������ֳ�Ī���Σ���dz��ɫ���塣���ڿ����б�һ���������ȶ����dz��õ�Fe2+�Լ���ijʵ��С�����ù�ҵ����м��ȡĪ���Σ����ⶨ�䴿�ȡ�
��֪:��
��Ī�������Ҵ��ܼ������ܡ�
��Ī���ε���ȡ
�Է�����
��1������2�м��ȷ�ʽ ���ֱ�Ӽ��ȡ��p��ˮԡ���ȡ���ɳԡ��������������м����ʣ��ʱ�������ȹ��ˣ���ԭ���� ��
��2������3�а�����ʵ��������� ��
��3����ƷĪ��������� ϴ�ӣ�����ĸ��ţ���
a������ˮ b���Ҵ� c����Һ
��Ϊ�ⶨ���������(NH4)2SO4?FeSO4?6H2O���崿�ȣ�ijѧ��ȡm g�����������Ʒ���Ƴ�500 mL��Һ������������ɣ��ס��ҡ�����λͬѧ�������������ʵ�鷽������ش�
(��)����һ��ȡ20.00 mL�����������Һ��0.1000 mol��L��1������KMnO4��Һ�����ν��еζ���
(��)��������ȡ20.00 mL�����������Һ��������ʵ�顣
��1����ʵ���������ȷ��������һ�IJⶨ�������С�ڷ������������ԭ��Ϊ
����֤�Ʋ�ķ���Ϊ�� ��
(��)��������(ͨ��NH4+�ⶨ)ʵ�����ͼ������ʾ��ȡ20.00 mL�����������Һ���и�ʵ�顣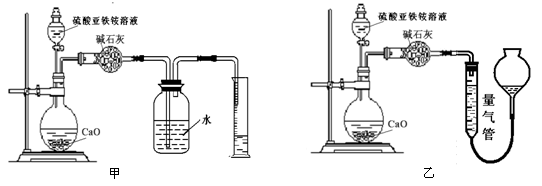
��2����װ�� ����ס����ҡ�����Ϊ�������ж������� ��������������Լ��� ������ĸ��š���ѡ���ҡ�����˿գ���ѡ���ס��˿տɲ����
a��ˮ b������NaHCO3��Һ c��CCl4
�������NH3�����ΪV L(������Ϊ��״����)�������������茶���Ĵ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ѡ��2-��ѧ�뼼������15�֣�
���ð�Һ������ӡˢп��ʱ����ϡ���ḯʴп���õ��ġ���Һ��(����������Cl����Fe3��)��ij��ѧ��ȤС�����á��ð�Һ����ȡZn(NO3)2��6H2O�Ĺ������£� 
��֪��Zn(NO3)2��6H2O��һ����ɫ���壬ˮ��Һ�����ԣ�Zn(NO3)2����Ӧ���õ��IJ���������ԡ�
��1�����ð�Һ�������ʵ���Ҫ�ɷ���________(�ѧʽ)����ϡ���ḯʴп�����������ΪN2O��д��ϡ���ḯʴп�巴Ӧ����Ҫ��ѧ����ʽ______________________��
��2���ڲ������б���pH��8��Ŀ����_________________________________��
��3�����������Ҫ�ɷ���___________________________________________��
��4���������м��ȡ���е�Ŀ����___________���˲������������������_______________��
��5�������ܱ���pH��2��Ŀ����_______���˲�����������õ���Ҫ������_____ ______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijУѧ����ѧʵ��С��,Ϊ��֤�ǽ���Ԫ���ȵ�������ǿ����͵�,�����һ��ʵ��װ��(���ּг�װ������ȥ):
(1)д��A�з�Ӧ�����ӷ���ʽ����������������������������������
(2)B�г��ֻ�ɫ��������,��������������ӷ���ʽ������������������������������
(3)�Դ�ԭ�ӽṹ�ǶȽ����ȵ������Դ������ԭ��������������������������
(4)D�и�����г��ֵ�����ѧ����ʽ����������������������������������
(5)��ͬѧ��ΪD�е�������˵���ȵ������Դ��ڵ�,��Ҫ��C֮ǰ��װϴ��װ��,�뻭����װ��ͼ������������(��ע��ʢװ�Լ�)��
(6)����ʲô������֤��������Cl2>S,��һ�������ʵ˵������������������������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com