
��ϩ����Ҫ�Ļ���ԭ�ϣ����Ժϳɺܶ��л������ת����ϵ��A-J��Ϊ�л������HΪ�з�����ζ��Һ�壬F�ķ���ʽΪC2H2O2��I��������һ����ԭ�ӻ�״�ṹ�����ַ�Ӧ����������������ȥ��
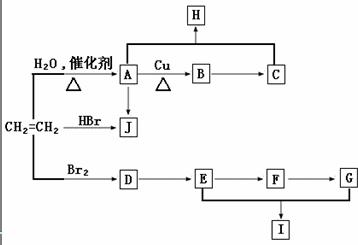
����������⣺
��1��д��A����B�Ļ�ѧ����ʽ��
��2��д��A����J�Ļ�ѧ����ʽ��
��3��д��D����E�Ļ�ѧ����ʽ��
��4��д��E��G����I�Ļ�ѧ����ʽ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��B��C��D��E����Ԫ�أ����ǵ��������������ࡣ
��A�ĺ˵��������2�����γ��⻯��H2A�����⻯���ڳ�������Һ�壻
��A��B��Ԫ�ؿ��γ�B2A3������û������������ǿ�ᣬ��������ǿ�
��C����B3����8�����ӣ�
��C��DԪ�ؿ����γɻ�����CD��
��CD����Һ��ͨ��������ӵ�����Һ����ɫ��
�������ڱ���E����C�����������ڣ�E���ʿ�����ˮ��Ӧ������������ӦʱE�ĵ��������ɵ����������ʵ���֮��Ϊ2��1���Իش�
(1) E��________(дԪ�ط���)��
(2)B�����ӽṹʾ��ͼ____________�� CԪ������������Ӧ��ˮ����ĵ���ʽ________��
(3)�õ���ʽ��ʾH2A�γɹ��̣�____________________ _________________��
(4)д��CD����Һ��ͨ�����������ӷ���ʽ��_______________ _______________��
(5)�Ƚ�B��C��E����Ԫ���γɵļ����������Ե�ǿ����(B��C��E������ʵ�����ӷ��ű�ʾ)�����ԣ�________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͬλ���� ��ԭ�Ӻ��ڵ�����������������֮���ǣ�������
��ԭ�Ӻ��ڵ�����������������֮���ǣ�������
��A.32��������B.67��������C.99��������D.166
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����
A��������̼������Һ��Ӧ��
2H+��CO32��== CO2����H2O
B��������Һ������������ͭ��Ӧ��
CH3COOH��OH�� �� CH3COO����H2O
C����������Һ��ͨ������������̼��
2C6H5O����CO2��H2O �� 2C6H5OH��CO32��
D����ȩ��Һ��������������Һ���ȣ�
HCHO+4[Ag(NH3)2]++4OH-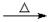 CO32��+2NH4++4Ag��+6NH3+2H2O
CO32��+2NH4++4Ag��+6NH3+2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ʱȽ��У���ȷ���� �� ��
�����ԣ�HClO4>HBrO4>HIO4 �ڼ��ԣ�Ba(OH)2>Mg(OH)2>Be(OH)2
�������ԣ�F>C>O �ܻ�ԭ�ԣ�Cl<S<Si
����̬�⻯���ȶ��ԣ�HF��HCl��H2S
A.�٢ڢ� B.�ڢۢ� C.�٢ڢܢ� D.�٢ڢۢܢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и��������л�ѧ����������ͬ���� ( )
A��CaCl2 MgCl2 Na2O B��H2O Na2O CO2
C��CaCl2 NaOH H2SO4 D��NH4Cl H2O CO2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���о���ѧ��Ӧ�е������仯ʱ������ͨ���������ʵ�飺
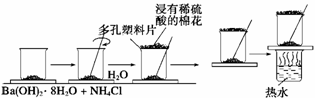
��һ��С�ձ������20 g����ĥ�ɷ�ĩ��Ba(OH)2��8H2O����С�ձ����������ѵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ�����ձ��м���Լ10 g NH4Cl���壬����ʵ�鲽�裬��д�±������ش����⡣
| ʵ�鲽�� | ʵ�������� |
| �������ϣ��������ٽ��� | �д̼�����ζ��ʹʪ�����ɫʯ����ֽ������___��__���� |
| �������ձ��²� | �о��ձ�����˵���˷�Ӧ��_ �� ��Ӧ |
| ���������ձ� | �ձ�����Ĵ��м���ˮ�IJ���Ƭճ�����ձ��ײ� |
| ��ճ�в���Ƭ���ձ�����ʢ����ˮ���ձ��� | ����Ƭ���������ձ��ײ� |
| ��Ӧ�����߶������Ƭ�۲췴Ӧ�� | �����ɺ�״��֤����_��___���� |
��1��д����Ŀ�Т٢ڢ����������
�� �� ��
��2��ʵ����Ҫ�����ò�����Ѹ�ٽ����ԭ���ǣ� __________________����2�֣�
��3��������ʵ������У�Ϊʲô�ý���ϡ�����ʪ�����ڶ�����ϰ��ϣ���2�֣�
___________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��һ�ֹ裬����������ȡ����������Ʒ�ֱ�Ͷ��������ϡ�����������ϡ����������Һ�У��ų�������H2����ôֹ�������Ĺ�ϵ��ȷ����(��ʾ��Si��2NaOH��H2O===Na2SiO3��2H2��) (����)��
A�����ʵ���֮��Ϊ��1��1 B�����ʵ���֮��Ϊ1��2
C������֮��Ϊ4��1 D������֮��Ϊ2��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com