
،¾جâؤ؟،؟ؤ³سذ»ْ»¯؛دخïAµؤدà¶ش·ض×سضتء؟ذ،سع200،£¾·ضخِµأضھ£¬ئنضذج¼µؤضتء؟·ضتخھ64.52%£¬اâµؤضتء؟·ضتخھ9.68%£¬ئنسàخھرُ،£A¾كسذثلذش£¬تا·نحُ½¬ضذµؤسذذ§³ة·ض£¬37.2 mg A ذèزھ20.0 mL 0.010 0mol،¤L-1 اâرُ»¯ؤئث®بـز؛ہ´µخ¶¨´ïµ½ضصµم،£
£¨1£©سذ»ْ»¯؛دخïAµؤدà¶ش·ض×سضتء؟تا_______________________،£
£¨2£©¸أ»¯؛دخïµؤ·ض×ست½تا_______________________،£
£¨3£©¸أ·ض×سضذ؛¬سذ__________¸ِôب»ù£¬حئ¶د¹³ج£؛__________________________________،£
،¾´ً°¸،؟186C10H18O31n(C10H18O3)=![]() =2،ء10-4mol£¬n(NaOH)= 2،ء10-4mol£¬AسëNaOH°´خïضتµؤء؟ض®±ب1:1·´س¦£¬ثùزش¸أسذ»ْخï·ض×س½ل¹¹ضذ؛¬سذز»¸ِôب»ù،£
=2،ء10-4mol£¬n(NaOH)= 2،ء10-4mol£¬AسëNaOH°´خïضتµؤء؟ض®±ب1:1·´س¦£¬ثùزش¸أسذ»ْخï·ض×س½ل¹¹ضذ؛¬سذز»¸ِôب»ù،£
،¾½âخِ،؟
£¨1£©¸أسذ»ْخïتاسةC،¢H،¢Oبضضشھثط×é³ة£¬زٍ´ثرُµؤضتء؟·ضتخھ(1£64.52%£6.68%)=25.8%£¬C،¢H،¢Oµؤش×س¸ِت±بخھ64.52%/12£؛9.68%/1£؛25.8%/16=10£؛8£؛3£¬¸أسذ»ْخïµؤ×î¼ٍت½خھC10H8O3£¬زٍخھAµؤدà¶ش·ض×سضتء؟ذ،سع200£¬زٍ´ثسذ(C10H8O3)nµؤدà¶ش·ض×سضتء؟ذ،سع200£¬¼´n=1£¬¸أسذ»ْخï·ض×ست½خھC10H8O2£¬دà¶ش·ض×سضتء؟خھ186£»£¨2£©¸ù¾ف£¨1£©µؤ·ضخِ£¬¸أسذ»ْخï·ض×ست½خھC10H8O2£»£¨3£©A¾كسذثلذش£¬ثµأ÷؛¬سذôب»ù£¬¸ù¾فAµؤ·ض×ست½£¬1mol¸أسذ»ْخï؛¬سذ3molO£¬زٍ´ث¸أسذ»ْخïضذ؛¬سذ1¸ِôب»ù£¬»ٍصكn(C10H18O3)=![]() =2،ء10-4mol£¬n(NaOH)= 2،ء10-4mol£¬AسëNaOH°´خïضتµؤء؟ض®±ب1:1·´س¦£¬ثùزش¸أسذ»ْخï·ض×س½ل¹¹ضذ؛¬سذز»¸ِôب»ù،£
=2،ء10-4mol£¬n(NaOH)= 2،ء10-4mol£¬AسëNaOH°´خïضتµؤء؟ض®±ب1:1·´س¦£¬ثùزش¸أسذ»ْخï·ض×س½ل¹¹ضذ؛¬سذز»¸ِôب»ù،£



| ؤ꼶 | ¸كضذ؟خ³ج | ؤ꼶 | ³ُضذ؟خ³ج |
| ¸كز» | ¸كز»أâ·ر؟خ³جحئ¼ِ£، | ³ُز» | ³ُز»أâ·ر؟خ³جحئ¼ِ£، |
| ¸ك¶ | ¸ك¶أâ·ر؟خ³جحئ¼ِ£، | ³ُ¶ | ³ُ¶أâ·ر؟خ³جحئ¼ِ£، |
| ¸كب | ¸كبأâ·ر؟خ³جحئ¼ِ£، | ³ُب | ³ُبأâ·ر؟خ³جحئ¼ِ£، |
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟¸ù¾فدآءذ¸÷ح¼اْدك±يص÷µؤذإد¢£¬µأ³ِµؤ½لآغ²»صب·µؤتا


A. ح¼1±يت¾³£خآدآدٍجه»خھ10 mL 0.1 mol،¤L£1NaOHبـز؛ضذضًµخ¼سبë0.1 mol،¤L£1CH3COOHبـز؛؛َبـز؛µؤpH±ن»¯اْدك£¬شٍbµم´¦سذ£؛c(CH3COOH)£«c(H£«)£½c(OH£)
B. ح¼2±يت¾سأث®د،تحpHدàح¬µؤرخثل؛ح´×ثلت±بـز؛µؤpH±ن»¯اْدك£¬ئنضذ¢ٌ±يت¾´×ثل£¬¢ٍ±يت¾رخثل£¬ازبـز؛µ¼µçذش£؛c>b>a
C. ح¼3±يت¾H2سëO2·¢ةْ·´س¦¹³جضذµؤؤـء؟±ن»¯£¬H2µؤب¼ةصببخھ285.8 kJ،¤mol£1
D. سةح¼4µأ³ِبô³ب¥CuSO4بـز؛ضذµؤFe3£«£¬؟ة²ةسأدٍبـز؛ضذ¼سبëتتء؟CuO£¬µ÷½عبـز؛µؤpHضء4×َسز
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟ؤ³»¯ر§ذ،×é²ةسأبçح¼ثùت¾×°ضأ£¬¶شإ¨دُثلسëؤ¾ج؟µؤ·´س¦½ّذذج½¾؟(زرضھ£؛4HNO34NO2،ü£«O2،ü£«2H2O)،£

اë»ط´ًدآءذختجâ£؛
(1)¼ى²é×°ضأئّأـذش؛َ£¬½«ب¼ةص³×ضذµؤؤ¾ج؟شع¾ئ¾«µئةد¼سببضء؛ىبب×´ج¬£¬ةىبëب؟عةصئ؟ضذ£¬²¢بû½ôئ؟بû£¬µخ¼سإ¨دُثل£¬؟ة¹غ²ىµ½ب؟عةصئ؟ضذئّجهµؤرصة«خھ________£¬²ْةْ¸أئّجهµؤ»¯ر§·½³جت½تا____________،£
(2)×°ضأCضذت¢سذ×مء؟Ba(OH)2بـز؛£¬³مببµؤؤ¾ج؟سëإ¨دُثل·´س¦؛َ؟ة¹غ²ىµ½Cضذ³ِدض°×ة«³ءµي£¬¸أ°×ة«³ءµيخھ____________(جر§ت½)،£
(3)×°ضأBµؤ×÷سأتا__________________________،£
(4)×°ضأDضذتص¼¯µ½ءثخقة«ئّجه£¬²؟·ضح¬ر§بدخھتاNO£¬»¹سذ²؟·ضح¬ر§بدخھتاO2،£
¢ظدآءذ¶ش¸أئّجهµؤ¼ىرé·½·¨؛دتتµؤتا________،£
A£®³¨؟ع¹غ²ى×°ضأDضذ¼¯ئّئ؟ؤعئّجهµؤرصة«±ن»¯
B£®½«تھبَµؤہ¶ة«ت¯بïتشض½ةىب뼯ئّئ؟ؤع£¬¹غ²ىہ¶ة«ت¯بïتشض½تا·ٌ±ن؛ى
C£®½«´ّ»ًذاµؤؤ¾جُةىب뼯ئّئ؟ضذ£¬¹غ²ىؤ¾جُتا·ٌ¸´ب¼
¢عبç¹ûDضذ¼¯ئّئ؟ضذتص¼¯µؤخقة«ئّجهتارُئّ£¬شٍرُئّµؤہ´ش´تا________________،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟زرضھ´ïµ½µçہëئ½؛âµؤ0.1mol/Lµؤ´×ثلبـز؛ضذ£¬خھءث´ظ½ّ´×ثلµؤµçہ룬ح¬ت±ت¹بـز؛µؤpH½µµح£¬س¦²ةب،µؤ´ëت©تا
A.¼سبëز»¶¨ء؟µؤث®B.¼سبببـز؛C.¼سبëةظء؟رخثلD.¼سبë±ù´×ثل
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟CuSO4بـز؛ضذ؛¬سذةظء؟Fe3£«£¬؟ةح¨¹µ÷½عpHضµہ´½«Fe3£«³ب¥£¬دآءذتش¼ءضذسأہ´µ÷pHضµ×î¼رµؤتا£¨ £©
A.NaOHبـز؛B.إ¨°±ث®C.CuO¹ججهD.H2O2
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟ؤ³ضضµ°°×أ¸ؤـت¹µ°°×ضتث®½â£¬×÷سأµؤض÷زھ²؟خ»تاµ°°×ضتضذµؤëؤ¼ü£¬ز»°متاëؤ¼ü¶دءر،£¼ظبç¸أضضأ¸ز²ؤـت¹دآتِخïضتث®½â£¬اë»ط´ًدآءذختجâ£؛
![]()
(1)¶دءرµؤ¼üس¦خھ____________________________________£»
(2)ث®½â؛َذخ³ةµؤ²ْخïتا______________________________،£
(3)¸ù¾ف»¯ر§ضھت¶إذ¶دةدتِ²ْخï_____£¨جî،°تا،±»ٍ،°²»تا،±£©جىب»°±»ùثل£¬ہيسة£؛________،£
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟بçح¼تا½ًتôأ¾؛حآ±ثطµ¥ضت(X2)·´س¦µؤؤـء؟±ن»¯ت¾زâح¼،£دآءذثµ·¨صب·µؤتا
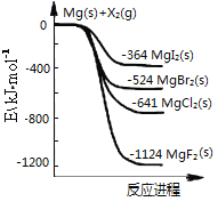
A. آ±ثطµ¥ضت(X2)سëث®·´س¦¾ù؟ةةْ³ةء½ضضثل
B. MgF2µؤµç×ست½خھ£؛![]()
C. ببخب¶¨ذش£؛MgI2£¾MgBr2£¾MgCl2£¾MgF2
D. سةح¼؟ةضھ´ثخآ¶بدآMgI2(s)سëCl2(g)·´س¦µؤبب»¯ر§·½³جت½خھ£؛MgI2(s)£«Cl2(g)=MgCl2(s)£«I2(g) ¦¤H£½£277 kJ،¤mol£1
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟¹طسعخïضتµؤ·ضہ࣬صب·µؤتا£¨ £©
A.ت¯»زث®£´؟¾»خ¼îت¯»ز£»ى؛دخï
B.ز؛آب£µ¥ضت£¬آبث®£»¯؛دخï
C.´؟¼î£صرخ£¬ذ،ثص´ٍ£ثلت½رخ
D.¶رُ»¯ج¼£ثلذشرُ»¯خ¹رُ»¯ؤئ£¼îذشرُ»¯خï
²é؟´´ً°¸؛ح½âخِ>>
؟ئؤ؟£؛¸كضذ»¯ر§ ہ´ش´£؛ جâذح£؛
،¾جâؤ؟،؟¸ù¾فدآءذتµرéدضدَ£¬ثùµأ½لآغصب·µؤتا( )
تµرé | تµرéدضدَ | ½لآغ | |
A |
| ×َةص±ضذجْ±يأوسذئّإف£¬سز±كةص±ضذح±يأوسذئّإف | رُ»¯ذش£؛Al3+>Fe2+>Cu2+ |
B |
| ×َ±كأق»¨±نخھ³بة«£¬سز±كأق»¨±نخھہ¶ة« | رُ»¯ذش£؛Cl2>Br2>I2 |
C |
| ×َ±كةص±ضذخقأ÷دش±ن»¯£¬سز±كةص±ضذ³خاهت¯»زث®±ن»ë×ا | ببخب¶¨ذش£؛Na2CO3>NaHCO3 |
D |
| ׶ذخئ؟ضذسذئّجه²ْةْ£¬ةص±ضذز؛جه±ن»ë×ا | ·ا½ًتôذش£؛Cl>C>Si |
A. AB. BC. CD. D
²é؟´´ً°¸؛ح½âخِ>>
¹ْ¼تر§ذ£سإر، - ء·د°²لءذ±ي - تشجâءذ±ي
؛±±ت،»¥ءھحّخ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨ئ½ج¨ | حّةدسذ؛¦ذإد¢¾ظ±¨×¨اّ | µçذإص©ئ¾ظ±¨×¨اّ | ةوہْت·ذéخقض÷زهسذ؛¦ذإد¢¾ظ±¨×¨اّ | ةوئَاضب¨¾ظ±¨×¨اّ
خ¥·¨؛ح²»ء¼ذإد¢¾ظ±¨µç»°£؛027-86699610 ¾ظ±¨ستدن£؛58377363@163.com