
��14�֣�ij�о�С�������������о���
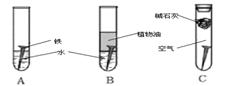
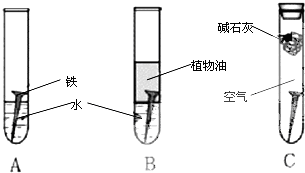
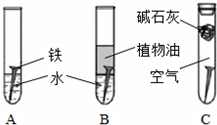
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ����
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ�� ��
��3����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��4����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
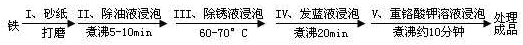
Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
��1��A ��3�֣� ��2��O2��2 H2O��4e���� 4 OH����3�֣�
��3��C ��3�֣� ��4������Ʒ�����к�ɫ����������3�֣�
����������1��ֲ��������ֹ��������ʯ��������CO2��ˮ��ʵ������������ʴ����A��
��2��ˮ�����Եģ���������������������ʴ��������ӦʽΪO2��2 H2O��4e���� 4 OH����
��3���Ʋ�������ʽ������ǿ�ڵ�����������ʹ�Ʋ����𣬷����绯ѧ��ʴ����Ҳ������������������˴�ѡC��
��4��������ϸ��������ܺ�����ͭ�����û���Ӧ�����ɺ�ɫ��ͭ���������������Ʒ�����к�ɫ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�긣��ʡ��Ϫһ�и�һ��ѧ����ĩ������ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣�ij�о�С�������������о���
��1�������ϳ�ʱ���ͬѧ�۲쵽�������ǣ���ͼ�е������������������ ������ĸ����
��2������ʵ�������жϣ�����������ʴ�����У������ĵ缫��ӦΪ�� ��
��3����Ϊ�˷�ֹ�������⣬��С��ͬѧ���������������һ��������ý��������
A. �� B. ͭ C. п
��4����������ʴ����ҵ�����г���Ը������С�����������������Ч�������������ĸ�ʴ����ν���������������ڸ�������Ƚ�������������ʹ������γ�һ�����ܵ�����ɫ����Ĥ�������������̿ɱ�ʾ���£�
Ϊ���龭������������������Ƿ�ϸ�����Ʒ�������5%������ͭ��Һ�������Ʒ���ϸ�����������С�ɿף�δ�γ����ܵ�����Ĥ����һ��ʱ�佫�۲쵽������Ϊ__________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com