
����˵����ȷ����(����)
A����һ���¶���AgClˮ��Һ�У�Ag����Cl��Ũ�ȵij˻���һ������
B��AgCl��Ksp��1.8��10��10 mol2��L��2�����κκ�AgCl�������Һ�У�[Ag��]��[Cl��]��Ag����Cl��Ũ�ȵij˻�����1.8��10��10 mol2��L��2
C���¶�һ��ʱ������Һ��Ag����Cl��Ũ�ȵij˻�����Kspֵʱ������ҺΪAgCl�ı�����Һ
D����AgClˮ��Һ�м������ᣬKspֵ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
V��W��X��Y��Z�������ڱ���1��20�Ų���Ԫ����ɵ�5�ֻ��������V��W��X��Z��Ϊ����Ԫ����ɡ�����5�ֻ������漰������Ԫ�ص�ԭ������֮�͵���35������֮��ķ�Ӧ��ϵ����ͼ��

��1��5�ֻ�����ֱ���V________��W________��X________��Y________��Z________��(�ѧʽ)
��2��������5�ֻ������е�ij2�ֻ����ﷴӦ������һ���»������������5�ֻ������е�����Ԫ�أ����ɸû�����Ļ�ѧ����ʽ��____________________________��
��3��V�ĵ���ʽ��________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �����о�����,�ø�Ĥ��ⷨ������Ũ����ȩ��ˮ���й������̼���Ľϵ͵��ŵ�,��ԭ����ʹ��ȩ�ֱ�����������������Ӧ,ת��Ϊ�Ҵ�������,�ܷ�ӦΪ2CH3CHO+H2O
�����о�����,�ø�Ĥ��ⷨ������Ũ����ȩ��ˮ���й������̼���Ľϵ͵��ŵ�,��ԭ����ʹ��ȩ�ֱ�����������������Ӧ,ת��Ϊ�Ҵ�������,�ܷ�ӦΪ2CH3CHO+H2O
CH3CH2OH+CH3COOH
ʵ������,��һ��Ũ�ȵ���ȩ-Na2SO4��ҺΪ�������Һ,ģ����ȩ��ˮ�Ĵ�������,��װ��ʾ��ͼ��ͼ��ʾ��
(1)���Լ���ȼ�ϵ��Ϊֱ����Դ,��ȼ�ϵ����b��Ӧͨ����������(�ѧʽ)���塣
(2)��������,�������ֱ�����������Ҵ���,��������ɫ���塣�缫��Ӧ����:
����:��4OH--4e-====O2��+2H2O
��
����:��
��CH3CHO+2e-+2H2O====CH3CH2OH+2OH-
(3)��������,������Na2SO4�����ʵ�����������(�������С�����䡱)��
(4)��֪:��ȩ���Ҵ��ķе�ֱ�Ϊ20.8�桢78.4�档�ӵ�������������Һ�з�����Ҵ���Ʒ�ķ������� ��
(5)��ʵ�ʹ��մ�����,��������ȩ��ȥ���ʿɴ�60%�������������ֱ�ע��1 m3��ȩ�ĺ���Ϊ3 000 mg��L-1�ķ�ˮ,�ɵõ��Ҵ���������kg(����������С�����1λ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ԡ�AgCl(s)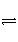
 Ag��(aq)��Cl��(aq)����������ȷ����(����)
Ag��(aq)��Cl��(aq)����������ȷ����(����)
��˵��AgClû����ȫ���룬AgCl���������
��˵���ܽ��AgCl����ȫ���룬��ǿ�����
��˵��Cl����Ag���ķ�Ӧ������ȫ���е���
��˵��Cl����Ag���ķ�Ӧ������ȫ���е��ס�������������������������������
A���ۢ� B���� C���٢� D���ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ����(����)
A���ܶȻ������ܽ�ƽ��ʱ���ܵ��������Һ�еĸ�����Ũ�ȵij˻�
B���ܶȻ������Dz����κ�����Ӱ��ij���������ܶȻ�
C������Ũ����Qc�жϳ����ܽ�ƽ����еķ���
D���������ʵ��ܶȻ��������¶ȵ����߶������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
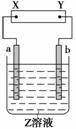
��ͼ��ʾ��X��Y�ֱ���ֱ����Դ��������ͨ�����a�����������ӣ�b���崦����ɫ����ζ����ų���������һ������Ǹ����е�(����)
| a���� | b���� | X�缫 | Z��Һ | |
| A | п | ʯī | ���� | CuSO4 |
| B | ʯī | ʯī | ���� | NaOH |
| C | �� | �� | ���� | AgNO3 |
| D | ͭ | ʯī | ���� | CuCl2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���84.75 mL 16%��NaOH��Һ(�ѣ�1.18 g·cm��3)����ʯī���缫����һ��ʱ�����ҺŨ��Ϊ19.5%��������Ϊ(����)
A������������3.5 g
B���ų���22.4 L H2��11.2 L O2
C��NaOH��ˮ������
D������18 gˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ȼ��ⱥ��ʳ��ˮ��ȡNaOH�Ĺ�������ʾ��ͼ���£�
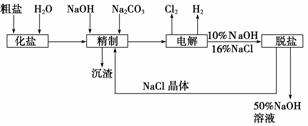
������ͼ�����������գ�
(1)�ڵ������У����Դ���������ĵ缫����������Ӧ�ĵ缫��ӦʽΪ________________________�����Դ���������ĵ缫��������ҺpH________(ѡ����䡱�������ߡ����½���)��
(2)��ҵʳ���к�Ca2����Mg2�������ʣ����ƹ����г�ȥ��Щ����ʱ������Ӧ�����ӷ���ʽΪ________________________________________________________________________��
________________________________________________________________________��
(3)���������SO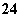 �������ߣ��������ӱ��Լ���ȥSO
�������ߣ��������ӱ��Լ���ȥSO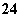 ���ñ��Լ�������________(��д��ĸ��ţ���ͬ)��
���ñ��Լ�������________(��д��ĸ��ţ���ͬ)��
A��Ba(OH)2����B��Ba(NO3)2����C��BaCl2
(4)Ϊ��Ч��ȥCa2����Mg2����SO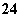 �������Լ��ĺ���˳��Ϊ________��
�������Լ��ĺ���˳��Ϊ________��
A���ȼ�NaOH�����Na2CO3���ټӱ��Լ�
B���ȼ�NaOH����ӱ��Լ����ټ�Na2CO3
C���ȼӱ��Լ������NaOH���ټ�Na2CO3
(5)���ι���������NaOH��NaCl���ܽ���ϵIJ��죬ͨ��________����ȴ��________
(��д��������)��ȥNaCl��
(6)�ø�Ĥ�����ʳ��ˮʱ�����۷ָ�Ϊ������������������ֹCl2��NaOH��Ӧ��������Ĥ��������ʳ��ˮʱ��Cl2��NaOH��ֽӴ����õ��IJ������NaClO��H2������÷�Ӧ��Ӧ�Ļ�ѧ����ʽΪ________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ��������)��
�ٷǽ���Ԫ�ع��ɵĵ�����һ�����ڹ��ۼ����ڷǽ���֮���γɵĻ�����һ���ǹ��ۻ�����۷ǽ�������̬�⻯����һ�����ڼ��Թ��ۼ��������ӻ�������һ���������Ӽ����ݽ���Ԫ�غͷǽ���Ԫ���γɵĻ�����һ�������ӻ��������һ�ֻ�������ֻ�ܴ���һ�����͵Ļ�ѧ�����ߺ����ۼ��Ļ����ﲻһ���ǹ��ۻ�����ຬ���Ӽ��Ļ�����һ�������ӻ�������Ȼ��ƺ�HCl����ˮ���������룬�˷����Ӽ���������������ͬ
A.�ۢܢߢ� B.�٢ۢܢߢ�
C.�٢ڢݢޢ� D.�٢ܢߢ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com