
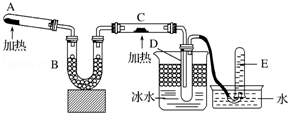
 ��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ��
��ѧ��һ����ʵ��Ϊ��������Ȼ��ѧ������ ��1�������������ζʱ������ֱ��ȥ�ţ�Ӧ����������ƿ���ȶ�����ʹ������������Ʈ���ǿף���ֹ�ж���
����ʵ���в����ж������ʵ��Ӧ����ͨ����н��в�����
��Na�Ϳ�����������ˮ��Ӧ��
������ƿֻ��������Һ�������ܽ��ϡ��ҩƷ��
��2��A��Ca��OH��2��NH4Cl��ϼ�����ȡ������B���ﰱ����C�а�����CuO�ڼ������������ɵ�����Cu��D����Һ��������������Һ������������ˮ��Ϊ��ˮ����ˮ���м��ԣ���ʹ��ɫʯ����ֽ���������еĽᾧˮ��ʹ��ˮ����ͭ������E�����ռ����ɵĵ�����
��ʵ��ʱ��C�а�����CuO�ڼ������������ɵ�����Cu��
���Թ�D���ռ����������ǰ�����������ʹʪ��ĺ�ɫʯ����ֽ����ɫ����ˮ����ͭ���Լ���ˮ��
��Mg���ڵ�����ȼ�գ�
��� �⣺��1�������������ζʱ������ֱ��ȥ�ţ�Ӧ����������ƿ���ȶ�����ʹ������������Ʈ���ǿף���ֹ�ж�������ȷ��
����ʵ���в����ж������ʵ��Ӧ����ͨ����н��в�����Cu��Ũ���ᷴӦ��ȡ�������������������ж����������ͨ����н��У��ײ����ж�������ȷ��
�����۵�ϵͣ���ȼ�գ��ƺ�ˮ��Ӧ���������ҷų������ȶ�ʹ�ƾ���ȼ�գ�����ʣ�����Ӧ�÷Ż�ԭƿ���ʴ���
������ƿֻ������һ�����ʵ���Ũ����Һ�������ܽ��ϡ��ҩƷ������ϡ����ʱ��Ҫ�Ƚ�Ũ�������ձ���ϡ�ͣ���ȴ�����º�ת�Ƶ�����ƿ�У�����ˮϡ�ͣ��ʴ���
��ѡ�٢ڣ�
��2��A��Ca��OH��2��NH4Cl��ϼ�����ȡ������B���ﰱ����C�а�����CuO�ڼ������������ɵ�����Cu��D����Һ��������������Һ������������ˮ��Ϊ��ˮ����ˮ���м��ԣ���ʹ��ɫʯ����ֽ���������еĽᾧˮ��ʹ��ˮ����ͭ������E�����ռ����ɵĵ�����
��ʵ��ʱ��C�а�����CuO�ڼ������������ɵ�����Cu����Ӧ����ʽΪ2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3H2O+3Cu���ʴ�Ϊ��2NH3+3CuO$\frac{\underline{\;\;��\;\;}}{\;}$N2+3H2O+3Cu��
�ڰ�����ԭ����֮ͭ��ʣ��İ����Ͳ�����ˮ����������ʱ���γɰ�ˮ�����а������Ӻͽᾧˮ�ijɷ֣���ˮ����ʹʹ��ɫʯ����ֽ���������еĽᾧˮ��ʹ��ˮ����ͭ������
�ʴ�Ϊ����ˮ���ú�ɫʯ����ֽ���飬��ֽ����������ˮ����ͭ���飬��ˮ����ͭ������
��Mg���ڵ�����ȼ�գ���Ӧ����ʽΪ3Mg+N2$\frac{\underline{\;��ȼ\;}}{\;}$Mg3N2�����Բ��ܣ�
�ʴ�Ϊ�����ܣ�þ�ڵ�����ȼ�գ�
���� ���⿼�黯ѧʵ�鷽�����ۼ���������ȡ�����ʣ�Ϊ��Ƶ���㣬��ȷʵ��ԭ����ʵ��������������ʵ������ǽⱾ��ؼ���ע��ͼ�и���װ�õ����ü����ܷ����ķ�Ӧ��֪�������ļ��鷽�������ʣ���Ŀ�ѶȲ���



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cu2++2OH-�TCu��OH��2�� CuCO3+2NaOH�TCu��OH��2��+Na2CO3 | |
| B�� | CO32-+2H+�TCO2��+H2O BaCO3+2HCl�TBaCl2+CO2��+H2O | |
| C�� | Ca2++CO32-�TCaCO3�� Ca��NO3��2+Na2CO3�TCaCO3��+2NaNO3 | |
| D�� | H++OH-�TH2O Ba��OH��2+H2SO4�TBaSO4��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���� | B�� | NaCl | C�� | NaOH | D�� | BaSO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na��O2 | B�� | Cu������ | C�� | Cu������ | D�� | Al��NaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | �г����Ŀ��� | B�� | ʳ��ˮ | C�� | ϡ���� | D�� | ˮ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaOH ��Һ�����ڴ����������Լ�ƿ�� | |
| B�� | ����FeCl2 ��Һʱ��ͨ������Һ�м�������Fe �� | |
| C�� | Cl2 ��SO2 ����Ư�����ã���Cl2 ��SO2 �������Ϻ��ʹƷ����Һ������ɫ | |
| D�� | �������ʵ���Ũ�ȵ���Һ������ʱ���ӿ̶���ʹ�����Ƶ���ҺŨ��ƫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
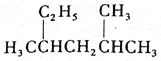 2��4-��������
2��4-��������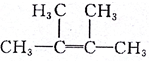 2��3-����-2-��ϩ
2��3-����-2-��ϩ�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ���ϲ��䵪�������� | |
| B�� | ѡ��20MPa-50MPa�ĸ�ѹ | |
| C�� | ��ʱҺ�����백�� | |
| D�� | ѡ��500�����ҵĸ���ͬʱʹ������ý������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
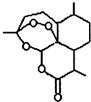 ����ʹ��ҩ���DZ�֤���Ľ��������������������Ч�ֶΣ�ҩ�ﻯѧ�Ѿ���Ϊ��ѧ��һ����Ҫ����
����ʹ��ҩ���DZ�֤���Ľ��������������������Ч�ֶΣ�ҩ�ﻯѧ�Ѿ���Ϊ��ѧ��һ����Ҫ����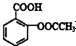 �dz��õĽ�����ʹҩ��������ˮ��������������Ӧ��ȡ����Ӧԭ��Ϊ��
�dz��õĽ�����ʹҩ��������ˮ��������������Ӧ��ȡ����Ӧԭ��Ϊ��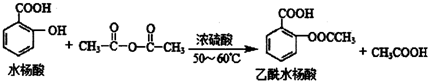
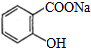 ��
���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com