
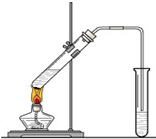
(7��) �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺

��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ����
��2���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ� ��
��3��Ũ����������ǣ��� ���� ��
��4������̼������Һ����Ҫ������
��
��5����Ҫ���Ƶõ������������������Ӧ���õ�ʵ��������� ������������
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺| Ũ���� |
| �� |
| Ũ���� |
| �� |
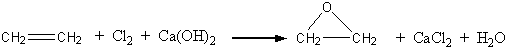
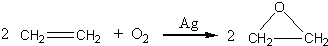
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
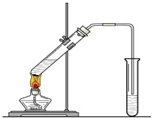 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ����������ͼ��ʾ��װ����ȡ�����������ش��������⣺| Ũ���� |
| �� |
| Ũ���� |
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 �����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
�����dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺ CH3CO18OCH2CH3+H2O
CH3CO18OCH2CH3+H2O CH3CO18OCH2CH3+H2O
CH3CO18OCH2CH3+H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ�ͷ33�и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(10�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺
��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ ��������������
��2���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ� ��
��3��Ũ����������ǣ��� ���� ��
��4������̼������Һ����Ҫ������
��5��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ�� ��
��6����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������������������� ����
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ɹŸ�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
(10�֣������dzµ��㡱��������Ϊ���ڴ������������������ζ��������������ʵ��������Ҳ��������ͼ��ʾ��װ����ȡ�����������ش��������⣺

��1��д����ȡ���������Ļ�ѧ��Ӧ����ʽ ��������������
��2���ڴ��Թ�������һ���������Ҵ��������Ũ����Ļ��Һ�ķ����ǣ� ��
��3��Ũ����������ǣ��� ���� ��
��4������̼������Һ����Ҫ������
��5��װ����ͨ�����ĵ���Ҫ���ڱ���̼������Һ��Һ���ϣ����ܲ�����Һ�У�Ŀ���Ƿ�ֹ�� ��
��6����Ҫ���Ƶõ������������������Ӧ���õ�ʵ������������������� ����
��7������ʵ��ʱ����ʱ����ʢ������Ҵ����Թ�����뼸�����Ƭ����Ŀ���� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com