ѡ����
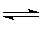
���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣
�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl
3��ΪFe
2(SO
4)
3��Ϊ��������������__________________��д��H
2O
2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ ________________________��
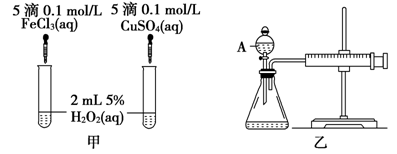
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������________________�������װ�������Եķ�����___________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ�� 2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol/(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯 B����������ܶȲ�����ʱ����仯 C��A���������ٸı� D��ƽ���������ƽ����Է����������ٸı�

 C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________  �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�