
��ӦaM(g����bN(g�� cP(g����d Q(g���ﵽƽ��ʱ��M���������y(M���뷴Ӧ�����Ĺ�ϵ��ͼ��ʾ������z ��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȡ�����˵������ȷ���ǣ� ��
cP(g����d Q(g���ﵽƽ��ʱ��M���������y(M���뷴Ӧ�����Ĺ�ϵ��ͼ��ʾ������z ��ʾ��Ӧ��ʼʱN�����ʵ�����M�����ʵ���֮�ȡ�����˵������ȷ���ǣ� ��

A��ͬ��ͬѹͬzʱ�����������ƽ��ʱQ������������ı�
B��ͬ��ͬѹʱ������z��ƽ��ʱQ���������һ������
C��ͬ��ͬzʱ������ѹǿ��ƽ��ʱQ���������һ����С
D��ͬѹͬzʱ�������¶ȣ�ƽ��ʱQ���������һ������
 ��ʦ����ָ���ο�ʱϵ�д�
��ʦ����ָ���ο�ʱϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и߶��ϵڶ����¿���ѧ���������棩 ���ͣ������
CO���ִ����������Ļ���ԭ�ϣ������й����ⶼ��CO��ʹ���йء�
��1�� ��ҵ�Ͽ�����CO�����Ҵ�:
2CO��g����4H2��g�� CH3CH2OH��g����H2O��g�� ��H1
CH3CH2OH��g����H2O��g�� ��H1
����֪��H2O��l��=== H2O��g�� ��H2
CO��g����H2O��g�� CO2��g����H2��g�� ��H3
CO2��g����H2��g�� ��H3
��ҵ��Ҳ������CO2��g����H2��g��Ϊԭ�Ϻϳ��Ҵ���
2CO2��g����6H2��g�� CH3CH2OH��g����3H2O��l�� ��H
CH3CH2OH��g����3H2O��l�� ��H
��H�릤H1����H2����H3֮��Ĺ�ϵ�ǣ���H��___________________��
��2��һ�������£�H2��CO������̶����ܱ������з������·�Ӧ��
4H2��g��+2CO��g�� CH3OCH3��g��+H2O��g��������ѡ�����жϸ÷�Ӧ�ﵽƽ��״̬�����ݵ���___ ��
CH3OCH3��g��+H2O��g��������ѡ�����жϸ÷�Ӧ�ﵽƽ��״̬�����ݵ���___ ��
A��2v��H2��= v��CO��
B��CO���������ʵ���CH3OCH3����������
C�������ڵ�ѹǿ���ֲ���
D�����������ܶȱ��ֲ���
E����������ƽ����Է�����������ʱ����仯
��3����ҵ�ɲ���CO��H2��Ӧ�ϳ�������Դ�״�����Ӧ����:
CO��g��+ 2H2��g�� CH3OH��g����һ�ݻ��ɱ���ܱ������г���10molCO��20molH2���ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��p���Ĺ�ϵ�磨ͼ1����ʾ��
CH3OH��g����һ�ݻ��ɱ���ܱ������г���10molCO��20molH2���ڴ��������·�����Ӧ���ɼ״���CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��p���Ĺ�ϵ�磨ͼ1����ʾ��

�ٺϳɼ״��ķ�ӦΪ ������ȡ������ȡ�����Ӧ��
��A��B��C�����ƽ�ⳣ��KA��KB��KC�Ĵ�С��ϵΪ ��
�����ﵽƽ��״̬Aʱ�����������Ϊ10L������ƽ��״̬Bʱ���������Ϊ_________L��
�ܣ�ͼ2��������Ϊ�÷�Ӧ��ʹ�ô��������¹�����ʼ������COͶ�ϱȺ�COƽ��ת���ʵĹ�ϵͼ�� ����������ȫ��ͬʱ����ʵ������ʹ�ô��������COƽ��ת���ʵ�ʾ��ͼ��

��CO��ƽ��ת���ʣ��������¶ȣ�T����ѹǿ��p���Ĺ�ϵ�磨ͼ3����ʾ��ʵ������ʱ����������250 �桢 1.3��104 kPa���ң�ѡ���ѹǿ��������__________________��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и߶��ϵڶ����¿���ѧ���������棩 ���ͣ�ѡ����
��ͬ�¶��£������Ϊ1.5 L���������������з������淴Ӧ��X2 (g��+3Y2(g��  2XY3(g�� ��H=��92.6 kJ��mol��1��ʵ�����й��������±���
2XY3(g�� ��H=��92.6 kJ��mol��1��ʵ�����й��������±���
������� | ��ʼʱ���������ʵ���/mol | ��ƽ��ʱ��ϵ�����ı仯 | ||
X2 | Y2 | XY3 | ||
�� | 1 | 3 | 0 | ����46.3 kJ |
�� | 0.8 | 2.4 | 0.4 | Q��Q��0�� |
������������ȷ���� �� ��
A���������дﵽƽ��ʱ��Y2��ת����Ϊ50%
B��Q=27.78 kJ
C����ͬ�¶��£���ʼʱ�������г���1.0 X2 mol ��3.0 mol Y2��2 mol XY3����Ӧ�ﵽƽ��ǰv(����>v(�棩
D�������١����з�Ӧ��ƽ�ⳣ����ȣ�K=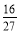
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ�����и߶��ϵڶ����¿���ѧ���������棩 ���ͣ�ѡ����
��֪��
2H2(g����O2(g��===2H2O(l�� ��H����571.6 kJ��mol��1
2CH3OH(l����3O2(g��===2CO2(g����4H2O(l�� ��H����1452 kJ��mol��1
H��(aq����OH��(aq��===H2O(l�� ��H����57.3 kJ��mol��1
����˵����ȷ���� �� ��
A��H2(g����ȼ����Ϊ571.6 kJ��mol��1
B��ͬ������H2(g����CH3OH(l����ȫȼ�գ�H2(g���ų���������
C�� H2SO4(aq����
H2SO4(aq���� Ba(OH��2(aq��===
Ba(OH��2(aq��=== BaSO4(s����H2O(l�� ��H����57.3 kJ��mol��1
BaSO4(s����H2O(l�� ��H����57.3 kJ��mol��1
D��3H2(g����CO2(g��=CH3OH(l����H2O(l�� ��H����135.9 kJ��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�������տ�������һ����ѧ���������л�ѧ�Ծ��������棩 ���ͣ�ѡ����
Ϊ��ǿ������ʴ�ԣ�����Ǧ����Ϊ���Դ����Al��������Pb�����������ϡ���ᣬʹ�����������Ĥ�����䷴Ӧԭ�����£�
��أ�P b��s��+PbO2��s��+2H2SO4��aq���T2PbSO4��s��+2H2O��l��
b��s��+PbO2��s��+2H2SO4��aq���T2PbSO4��s��+2H2O��l��
���أ�2Al+3H2O Al2O3+3H2���������У������ж���ȷ���ǣ� ��
Al2O3+3H2���������У������ж���ȷ���ǣ� ��
��� | ���� | |
A | H������Pb�缫 | H������Pb�缫 |
B | ÿ����3molPb | ����2molAl2O3 |
C | ������PbO2+4H��+2e�TPb2++2H2O | ������2Al+3H2O?6e?�TAl2O3+6H�� |
D |
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017������ʡ����10�¼�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ������SO2ͨ��FeC13��Һ�У�ȡ�����Һ���ֱ��������ʵ�飬��֤��SO2��FeCl3��Һ����������ԭ��Ӧ���ǣ� ��
ѡ�� | ���� | ���� |
A | ����NaOH ��Һ | �к��ɫ���� |
B | ����Ba ��NO3��2��Һ | �а�ɫ���� |
C | ��������KMnO4��Һ | ��ɫ��ȥ |
D | ����KSCN��Һ | ���������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com