
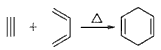 �����Ҫ�ϳ�
�����Ҫ�ϳ�  ���õ�ԭʼԭ�Ͽ�����__________
���õ�ԭʼԭ�Ͽ�����__________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��һ��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ��
��һ��ѭ���ֽ�ˮ������Ҫ�漰���з�Ӧ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
����I����ȡ0.4 g��������Ʒ��������������ĵ�ƿ(��ͼ)�ڣ�����10 mL���Ȼ�̼������ҡ��
ʹ��ȫ���ܽ⡣���ƿ�м���25.00 mL��0.01 molIBr����ˮ������Һ���Ǻ�ƿ�����ڲ�������ƿ��֮��μ�����10���⻯����Һ��շ�϶������IBr�Ļӷ���ʧ��
����II���ڰ�������30 min������ʱ����ҡ����30 min��С�ĵش��������������Ƶ�10��
�⻯��10 mL������ˮ50 mL�Ѳ�������ƿ���ϵ�Һ���ϴ��ƿ�ڡ�
�������ָʾ������0.1 mol��L��1�����������Һ�ζ���������ƿ��ֱ���յ㡣
�ⶨ�����з�������ط�Ӧ���£�
�� ��IBr��KI=I2+KBr �� I2��2S2O32��=2I����S4O62��
��ش��������⣺
(1)��֪±�ػ�����IBr��������±�ص������ƣ�ʵ����ȷ��ȡIBr��ҺӦѡ�õ������� ����ƿ������ᷢ����Ӧ�Ļ�ѧ����ʽ ��
(2)������е�ƿ�ڰ�������30 min������ʱ����ҡ����ԭ���� ��
(3)�����������ָʾ��Ϊ ���ζ��յ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ���Ĵ�ʡ�ϳ��и����ڶ�������Կ��ԣ����ۣ���ѧ���� ���ͣ������
��ҵ�ϳɰ����Ʊ�����һ�����������,������ͼ17��ʾ��

��ش��������⣺
I.�ϳɰ�
(1)��֪��һ�����¶��½���װ�âٵĵ�����������(�����ۼ����Ȼ�ϣ����װ�âٳ����Ļ������ѹǿ֮��Ϊ5: 4��������ת����Ϊ________________________��
II.���ĽӴ�����ԭ��
��֪1 ����9000Cʱ��װ�â��з�Ӧ�У�

�������з�Ӧ�⣬����һ����������ã�
 ��
�� ,�����ܷ�������һ�������ķֽ⡣
,�����ܷ�������һ�������ķֽ⡣
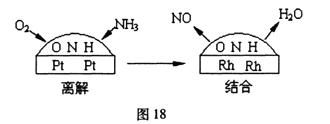
��֪2:��һ���Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ18��ʾ�����ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ��,ʹ��NO��ˮ�����ڲ������Ѹ�,���������С���ش��(2) (3)С�⣺

��2������Ȼ�ѧ����ʽ�� ��
�� =________________��
=________________��
��3����û��ʹ����һ��Ͻ����,�������������Ҫ����________��˵�������Է�Ӧ��________
��4���¶ȶ�һ���������ʵ�Ӱ�죨ͼ19)

���¶ȴ���900¾ʱ��NO�IJ����½���ԭ��________________________(ѡ����ţ���
A.�ٽ���һ�������ķֽ� B.�ٽ��˰��ķֽ�
C.ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳɰ����Ʊ�����һ�����������,������ͼ17��ʾ��

��ش��������⣺
I.�ϳɰ�
(1)��֪��һ�����¶��½���װ�âٵĵ�����������(�����ۼ����Ȼ�ϣ����װ�âٳ����Ļ������ѹǿ֮��Ϊ5: 4��������ת����Ϊ________________________��
II.���ĽӴ�����ԭ��
��֪1 ����9000Cʱ��װ�â��з�Ӧ�У�
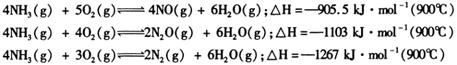
�������з�Ӧ�⣬����һ����������ã�
![]() ��
��![]() ,�����ܷ�������һ�������ķֽ⡣
,�����ܷ�������һ�������ķֽ⡣
��֪2:��һ���Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ18��ʾ�����ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ��,ʹ��NO��ˮ�����ڲ������Ѹ�,���������С���ش��(2) (3)С�⣺
��2������Ȼ�ѧ����ʽ��![]() ��
��![]() =________________��
=________________��
��3����û��ʹ����һ��Ͻ����,�������������Ҫ����________��˵�������Է�Ӧ��________
��4���¶ȶ�һ���������ʵ�Ӱ�죨ͼ19)

���¶ȴ���900??ʱ��NO�IJ����½���ԭ��________________________(ѡ����ţ���
A.�ٽ���һ�������ķֽ� B.�ٽ��˰��ķֽ�
C.ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ҵ�ϳɰ����Ʊ�����һ�����������,������ͼ17��ʾ��
��ش��������⣺
I.�ϳɰ�
(1)��֪��һ�����¶��½���װ�âٵĵ�����������(�����ۼ����Ȼ�ϣ����װ�âٳ����Ļ������ѹǿ֮��Ϊ5: 4��������ת����Ϊ________________________��
II.���ĽӴ�����ԭ��
��֪1 ����9000Cʱ��װ�â��з�Ӧ�У�
�������з�Ӧ�⣬����һ����������ã�
��
,�����ܷ�������һ�������ķֽ⡣
��֪2:��һ���Ͻ�����Ĵ�����Ϊ���ͽ�������̣���ͼ18��ʾ�����ڲ���NO��ˮ���ӵ���������С�������ڵ�����ԭ�ӽ��,ʹ��NO��ˮ�����ڲ������Ѹ�,���������С���ش��(2) (3)С�⣺
��2������Ȼ�ѧ����ʽ����
=________________��
��3����û��ʹ����һ��Ͻ����,�������������Ҫ����________��˵�������Է�Ӧ��________
��4���¶ȶ�һ���������ʵ�Ӱ�죨ͼ19)
���¶ȴ���900¾ʱ��NO�IJ����½���ԭ��________________________(ѡ����ţ���
A.�ٽ���һ�������ķֽ� B.�ٽ��˰��ķֽ�
C.ʹ����һ�������ķ�Ӧƽ���ƶ������ɸ���N2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com