
ij����M����������Ľᾧˮ����M��OH��2��xH2O��Na2CO3�Ļ���ﹲ36.8g������������ˮ�����ɰ�ɫ�����������в����ᾧˮ�������������ˡ�ϴ�ӡ���ɣ��Ƶ�������Ϊ9.85g�����õ��ij����������պ�������Ϊ7.65g����Һ�������ò��������壬�����������������Һ���ȣ������4.48L���壨��״������
��1����Һ��n��OH����=____________mol
��2��M�����ԭ������_____________________
��3��M����������Ľᾧˮ����Ļ�ѧʽΪ____________________
��4����M�Ĵ�����������NH4Cl����ͼ��ʾװ�ã�����Ƭ�����ձ��ײ�֮����һ����ˮ���л�Ϸ�Ӧ�����������ձ�ʱ������Ƭ��___________________����÷�Ӧ��Ӧ�������仯��ϵͼ��_________
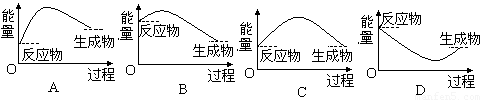
��1��0.2����2��137 ��3�� Ba��OH��2��8H2O ��4�� ���ձ�һ����������A
���������������ɵİ��������n��OH����=0.2mol/L����OH����ǰһ��û�в��뷴Ӧ����֪M��OH��2��xH2OΪ0.1mol/L������Һ�������ò���������֪M��OH��2��xH2O��Na2CO3ʱ������ȫ����Ӧ���ˡ��ֳ�����̼���Σ���MCO3 MO+CO2�������M�����ԭ������Ϊ137����M�DZ�Ԫ�ء������ɵ�9.85gBaCO3�����n��Na2CO3��=0.05mL���ٽ�ϻ���������������x=8��Ba��OH��2��8H2O��NH4Cl��Ӧʱ�����մ������ȶ�����ˮ��������ձ��벣��Ƭմ����һ�𣬸÷�Ӧ�����ȷ�Ӧ���������������ڷ�Ӧ�
MO+CO2�������M�����ԭ������Ϊ137����M�DZ�Ԫ�ء������ɵ�9.85gBaCO3�����n��Na2CO3��=0.05mL���ٽ�ϻ���������������x=8��Ba��OH��2��8H2O��NH4Cl��Ӧʱ�����մ������ȶ�����ˮ��������ձ��벣��Ƭմ����һ�𣬸÷�Ӧ�����ȷ�Ӧ���������������ڷ�Ӧ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)M������������NaHCO3��Ӧ�Ļ�ѧ����ʽΪ��?
����������������������������������������������?
(2)Ҫ����M�����ԭ������������Ϊ�������ṩ������������ (����ĸ���)��
A.M������������Һ�����ʵ���Ũ��(��Ϊ2 mol��L-1?)?
B.M��̼���ε�����(��Ϊ39.4 g)?
C.��M��̼���η�Ӧ����������ʵ���Ũ��(��Ϊ0.1 mol��L-1?)?
D.�������ݳ��㣬����Ҫ��������?
�������ѡ�������M�����ԭ������������������������?
(3)���������Һ��Ӧ����������ʵ���Ũ���� mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����M����������Ľᾧˮ����M(OH)2��xH2O��Na2CO3�Ļ���ﹲ36.800 g������������ˮ�����ɰ�ɫ����(���������ᾧˮ)���������˳���ϴ����ɣ��Ƶ�������Ϊ9.850 g�����õ��ij����������պ�������Ϊ7.650 g����Һ�������ò��������壻�����������������Һ���ȣ������4.48 L����(��״��)����
����Һ��OH�������ʵ���Ϊ__________mol��
����M��������Ϊ81����M��Ԫ�ط���Ϊ____________��
��M����������Ľᾧˮ����Ļ�ѧʽΪ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij����M����������Ľᾧˮ����M��OH��2��xH2O��Na2CO3�Ļ���ﹲ36.8g������������ˮ�����ɰ�ɫ�����������в����ᾧˮ�������������ˡ�ϴ�ӡ���ɣ��Ƶ�������Ϊ9.85g�����õ��ij����������պ�������Ϊ7.65g����Һ�������ò��������壬�����������������Һ���ȣ������4.48L���壨��״������
��1����Һ��n��OH����=____________mol
��2��M�����ԭ������_____________________
��3��M����������Ľᾧˮ����Ļ�ѧʽΪ____________________
��4����M�Ĵ�����������NH4Cl����ͼ��ʾװ�ã�����Ƭ�����ձ��ײ�֮����һ����ˮ���л�Ϸ�Ӧ�����������ձ�ʱ������Ƭ��___________________����÷�Ӧ��Ӧ�������仯��ϵͼ��_________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com