
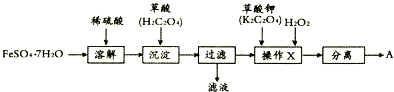
���� �Ʊ�������A��ʵ�����̣�FeSO4•7H2O�ܽ��������У��õ�����Һ�����������ӣ�����������ӣ������мӲ��ᣬ�õ������������������ˡ�ϴ�Ӻ���õ��IJ��������мӲ����H2O2���õ�������A��
��1���ٹ�����Ҫ�IJ����������ձ���©������������
�ڲ���X�õ�����������Ԫ��Ӧ��Ϊ+2�ۣ���������������������A����Ԫ��Ϊ+3�ۣ���H2O2�������ǰ�Fe2+ת��ΪFe3+��
��2��a��ȷ��ȡA��Ʒ4.91g��������ˮ�����أ����ٵ�����Ϊˮ��������n��H2O��=$\frac{4.91g-4.37g}{18g/mol}$=0.03mol��
b��������a���ù�������ˮ�����뻹ԭ����0.28g��������ѧ��Ӧ��Fe+2Fe3+=3Fe2+���ɵ�n��Fe3+����
c����ȡA��Ʒ4.91g������ƿ�У�����������3mol/L��H2SO4��Һ����������ˮ���ټ���0.50mol/L��KMnO4��Һ24.0mL���ȣ�ǡ����ȫ��Ӧ��2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O�ɼ���n��C2O42-�����������غ�֪n��K+������x��1��y��z=n��K+����n��Fe3+����n��C2O42-����n��H2O�����ݴ˷�����
��� �⣺��1��������Ҫ�IJ����������ձ���©�������������ʴ�Ϊ���ձ���©������������
���Ʊ�������A��ʵ�������е�FeSO4��H2C2O4�����л�ԭ�ԣ��ʲ���X�õ�����������Ԫ��Ӧ��Ϊ+2�ۣ���������A����Ԫ��Ϊ+3�ۣ���H2O2������Ӧ���ǰ�Fe2+ת��ΪFe3+������������
�ʴ�Ϊ����������
��2����a��ȷ��ȡA��Ʒ4.91g��������ˮ�����أ��������ʵ�����Ϊ4.37g�����ٵ�����Ϊˮ��������n��H2O��=$\frac{4.91g-4.37g}{18g/mol}$=0.03mol��
�ʴ�Ϊ��0.03��
��b��������a���ù�������ˮ�����뻹ԭ����0.28g��������ѧ��Ӧ��Fe+2Fe3+=3Fe2+����n��Fe3+��=2n��Fe��=2��$\frac{0.28g}{56g/mol}$=0.01mol��
c����ȡA��Ʒ4.91g������ƿ�У�����������3mol/L��H2SO4��Һ����������ˮ���ټ���0.50mol/L��KMnO4��Һ24.0mL���ȣ�ǡ����ȫ��Ӧ��
2KMnO4+5H2C2O4+3H2SO4=2MnSO4+K2SO4+10CO2��+8H2O��֪��n��C2O42-��=n��H2C2O4��=$\frac{5}{2}$n��KMnO4��=$\frac{5}{2}$��0.05mol/l��0.24L=0.03mol����A��Ʒ��$\frac{n��F{e}^{3+}��}{n��{C}_{2}{{O}_{4}}^{2-}��}$=0.01mol��0.03mol=1��3��
�ʴ�Ϊ��1��3��
���ɵ���غ�֪��n��K+��+3n��Fe3+��=2n��C2O42-����n��K+��+3��0.01mol=2��0.03mol��n��K+��=0.03mol��4.91g��ƷAKxFe��C2O4��y•zH2O�У�x��1��y��z=n��K+����n��Fe3+����n��C2O42-����n��H2O��=0.03mol��0.01mol��0.03mol��0.03mol=3��1��3��3��x=3��y=3��z=3����A�Ļ�ѧʽΪK3Fe��C2O4��3•3H2O��
�ʴ�Ϊ��K3Fe��C2O4��3•3H2O��
���� ���⿼�������ʵ��Ʊ���̽��������ɣ���Ŀ�Ѷ��еȣ�ע�����������غ㶨�ɡ�����غ��ڻ�ѧ�����е�Ӧ�÷���������������ѧ���ķ�����������ѧʵ�顢��ѧ����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | �Լ� | ���������� |
| A | ��ˮ | �μ�NaOH��Һ����Һ��ɫ��ȥ���ټ�Ũ���ᣬ��Һ��ɫ�ָ� |
| B | CaCl2 | ����֧ʢ��CaCl2��Һ���Թ��зֱ�ͨ��CO2��CO������������ |
| C | AgNO3 | �μӰ�ˮ�����������������μӰ�ˮ�������ܽ� |
| D | ���� | �μ�NaOH��Һ���������壬�����μӣ�������ɫ���� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 800mL0.5mol/L��NaCl��Һ | B�� | 100mL0.3mol/L��AlCl3��Һ | ||
| C�� | 500mL0.3mol/L��CaCl2��Һ | D�� | 300mL0.3mol/L��MgCl2��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| t/min | 0 | 3 | 10 | 12 |
| n��CO��/mol | 2 | 1 | 0.5 | 0.5 |
| n��CH3OH��/mol | 0 | 1 | 1.5 | 1.5 |
| A�� | ��0��3min�ڣ���H2��ʾ��ƽ����Ӧ����Ϊ0.33mol•L-1•min-1 | |
| B�� | �ڸ������£�������Ӧ��ƽ�ⳣ��Ϊ3 | |
| C�� | ��Ӧ��ƽ��ʱ��CH3OH ��g�����������Ϊ50% | |
| D�� | Ҫ����Ӧ���������� CH3OH ��g���ڻ�����е�����������ɲ���ѹ�������������ϵѹǿ�Ĵ�ʩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | AgCl��AgI��AgBr | B�� | AgCl��AgBr��AgI | C�� | AgBr��AgCl��AgI | D�� | AgBr��AgI��AgCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1��1 | B�� | 5��3 | C�� | 33��11 | D�� | 11��4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��պ��Ũ��ˮ���ް��������������Ĺܵ��Ƿ�©�� | |
| B�� | �з�ʹ�ø�Ч����������߷�Ӧ��ԭ�ϵ�ת���� | |
| C�� | ��K2FeO4ȡ��Cl2��������ˮ����ɱ�����������ܳ���ˮ�е������� | |
| D�� | �ߴ���㷺Ӧ����̫���ܵ�ء������оƬ�Ͱ뵼������������ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com