


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����
�������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ���ൺ�и����ڶ���ģ����ϰ ���ͣ������
��8�֣�����ѧ�����ʽṹ�����ʡ�
�� �������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����
������1��Cr�ĺ�������Ų�ʽΪ ��
��2����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£� 
ͼ�����߱�ʾ��������Ϊ ��
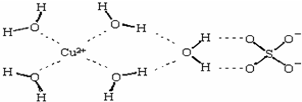
��3��������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣��Cu(NH3)4SO4��H2O�����У�[Cu(NH3)4]2+Ϊƽ�������νṹ�������������ṹ��ԭ������ ��������ԭ�ӵ��ӻ���������� ��
��4������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡����Ʋ����ʻ����ľ��������� �� �� Ni(CO)4���������� ��
| A��ˮ | B�����Ȼ�̼ | C������ | D����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ѧ�����ʽṹ�����ʡ�
�� �������ڵ�Cr��Fe��Co��Ni��Cu��Zn������������γ�����
������1��Cr�ĺ�������Ų�ʽΪ ��
��2����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£�
ͼ�����߱�ʾ��������Ϊ ��
��3��������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣��Cu(NH3)4SO4��H2O�����У�[Cu(NH3)4]2+ Ϊƽ�������νṹ�������������ṹ��ԭ������ ��������ԭ�ӵ��ӻ���������� ��
��4������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡����Ʋ����ʻ����ľ��������� �� �� Ni(CO)4 ���������� ��
A��ˮ ���� B�����Ȼ�̼ �� C���� �� D����������Һ
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com