
������16�֣���ijʵ��С��ͬѧΪ��̽��ͭ��Ũ����ķ�Ӧ������������ϵ��ʵ�顣
��ʵ��1��ͭ��Ũ���ᷴӦ��ʵ��װ����ͼ��ʾ��
ʵ�鲽�裺
�������Ӻ�װ�ã����������ԣ������Լ���
�ڼ���A�Թ�ֱ��B��Ʒ����ɫ��Ϩ��ƾ��ƣ�
�۽�Cu˿�����뿪Һ�档
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��Ϩ��ƾ��ƺ���Ϊ�е���D�Ĵ��ڣ�B�е�Һ�岻�ᵹ������ԭ���� ��
��3�����װ��ǰ������������Ϳ�ʹװ���в���������ȫ�����գ�Ӧ����ȡ�IJ����� ��
���͵�NaHSO3��Һ������ȩ�����ӳɷ�Ӧ������ˮ���ԵĦ�-�ǻ������ơ������ķ�ӦΪ��
R-CHO + NaHSO3 R-CH��OH��SO3Na
R-CH��OH��SO3Na
��Ӧ�ǿ���ģ���ͨ����������70%--90%������Ӧ����ת����
��4�����â���װ����ȡ����NaHSO3��Һ��Ӧ��ȡ��ʩ����װ�ý��в��ָı䡣���ִ�ʩ�ǣ�_____________________________________________________________________��ȷ����NaHSO3���ɵ�������:_______________________________________.
��5�����屽�л���������ȩ,���������ʳ�ȥ,�ɲ��õ��Լ���:__________,�����ķ�����:_____________________.
��6������CH3-CH(OH)SO3Na ˮ��Һ�м�����������,�л���ת��Ϊ:___________,����ת�������������ķ�������__________________.
��1��2H2SO4(Ũ)+Cu CuSO4+SO2��+2H2O (2��)
CuSO4+SO2��+2H2O (2��)
��2���Թ�A������ѹǿ��С��������D���ܽ����Թ�A�� (2��)
��3����D�ܿ���A�д������� (2��)
��4����B��C��λ�õ�����(2��)Ʒ����Һ��ɫ��(2��)
��5������NaHSO3��Һ(2��) ��Һ(2��) ��6��CH3CHO(2��)����(2��)
�������������
��1��Ũ������ͭ��Ӧ���ɲ��տα�Ũ��������ʡ�
��2��D�����������ǵ�A�е���ѹ��Сʱ����������D���ܽ���A�У���ֹ���������á�
��3��ͨ����Ŀ��֪����D�ܹ������ԴﵽĿ�ġ�
��4��Ҫ�Ʊ����������ƣ��ͱ���������������Һ��ͨ������Ķ�������ͬʱ��Ҫ����֤������������Ѿ��������뵽�����B��C���Թܵ����Ϳɣ����۲쵽Ʒ����Һ��ɫʱ��˵�����������Ѿ�����������������Ҳ�Ͳ����ˡ�
��5����С����Ҫ�����Ϣ����Ȼѡ�õ��DZ���������������Һ����ȥ����ȩ�����ڻ������ܵ�Һ�壬�����÷�Һ�ķ�����
��6����������������������Ƴ���ȩ��һ�����淴Ӧ����������������������Ƶ�Ũ���½���ƽ�����淴Ӧ�����ƶ���������ȩ����Ϊ��ȩ����ˮ��Һ���ʱ��Ҫ���÷е㲻ͬ��������ķ�����
���㣺Ũ��������ʣ�������������ʣ�������ᴿ�ķ�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��У��ѧ��ȤС���ѧ����ijƷ�Ƶ�����Һ�ijɷֺ����ʽ���ʵ��̽����
�ٸ�����Һ�����ɫ����ȡ�����μ�AgNO3��Һ���ɰ�ɫ�������ó������������ᣩ��
���ø���ྻ������պȡ����Һ���㵽pH��ֽ�ϣ���ֽ�ȱ�������ɫ��
��ȡ��������Һ���μ�ϡ������л���ɫ�������ɣ�
���ýྻ��˿պȡ����Һ������ɫ���������գ�����ʻ�ɫ��
��ȡ��������Һ��ͨ������H2S���壬�ȿ����С�dz��ɫ�����������֡����塱��ȡ������Һ�������μ�BaCl2��Һ���а�ɫ�������ɣ��ó������������ᣩ��
��ش��������⣺
��1��������Һ����Ҫ�ɷ���_______________________________________��
��2��pH��ֽ��ɫ�ı仯˵������Һ��Һ���е������� _________________��
��3��ʵ����е����ӷ���ʽΪ_______________________________________��
��4��ʵ����У��С�dz��ɫ����������ʱ�����ӷ���ʽΪ_________________________���֡����塱ʱ�����ӷ���ʽΪ__________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һ����������Ļ����Һ��ȡ��10mL��������BaCl2��Һ�����ˡ�ϴ�ӡ���ɺ�õ�9��32g�ij�������Һ��4mol��L-1NaOH��Һ��Ӧ����ȥ40mLNaOH��Һʱǡ����ȫ�к͡�����
��1�����Һ��H2SO4��HNO3�����ʵ���Ũ�ȸ��Ƕ��٣�
��2����ȡ10mLԭ���Һ������4��48gͭ�۹���ʱ���ռ��������ڱ�״���µ����Ϊ���ٺ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣�ijУ��ȤС������ͼ��װ����ȡƯ��Һ���������Ѽ��飬�Լ������ӣ������о���������ʡ�
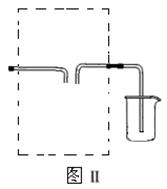
ʵ�����������Һ©���Ļ����������μ�һ����Ũ���ᣬ��ȼ�ƾ��ƣ�һ��ʱ��رշ�Һ©���Ļ�����Ϩ��ƾ��ơ�
��1����ƿ�з�Ӧ�Ļ�ѧ����ʽ�� ��
��2������ʳ��ˮ�������� ��
��3������ͼ��װ���ռ�������������������߿��ڻ�����װ�ü�ͼ��
��4���ķ�����С��ͬѧ�Ƶ��˽ϸ�Ũ�ȵ�NaClO��Һ�����ǰ�Ư��Һ�͵��з�̪�ĺ�ɫNa2SO3��Һ��Ϻõ���ɫ��Һ��
������룺
����NaClO��Na2SO3������ ����NaClO�ѷ�̪������
����NaClO��Na2SO3�ͷ�̪��������
������ʵ�鷽���п���֤��NaClO������Na2SO3���� ������ţ���
a�����Ϻ����Һ�м����������
b�����Ϻ����Һ�м���������ᣬ�ټ����Ȼ�����Һ
c�����Ϻ����Һ�м���������ᣬ�ټ�����������Һ
d�����Ϻ����Һ�м�������������Һ���ټ����������
��Ϊ֤��NaClO�����˷�̪���ɽ��е�ʵ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��16�֣���ԭ������������ƣ�Na2S2O3���ڹ�ҵ������ҽҩ����ҵ�б��㷺Ӧ�ã���ҵ�ձ�ʹ��Na2SO3����ǣ�S������õ���װ����ͼ1��
��֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1������1����K1���ر�K2����Բ����ƿ�м��������ײ����ȣ����Լ���Ϊ��
��
��2������2��ʼ�ձ���C����Һ�ʼ��ԣ���Ӧһ��ʱ�����۵������٣���K2���ر�K1��ֹͣ���ȡ�
��C����Һ�뱣�ֳʼ��Ե�ԭ���������ԣ��� ���� ������������ ��
���������ӷ���ʽ��ʾ��
��װ��B��D�������� ��
����3����C�����û��������ᴿ��ò�Ʒ��
��3�����÷�Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2Ҳ���Ʊ�Na2S2O3������������ͼ2���������������Ӹ��������ӿ�˳��Ϊ�� ��g��h�� �� �� �� ��d��
��4��װ����ʢװ���Լ��ǣ�_____________________________��
��5��Na2S2O3��ԭ�Խ�ǿ����ҵ�ϳ�������ȥ��Һ�в�����Cl2���÷�Ӧ�����ӷ���
ʽΪ�� ����
��6������Ƽ�ʵ�鷽����֤������������Cl2����ԭ����Cl����____________
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ����֤ľ̿�ɱ�ŨH2SO4������CO2��ѡ����ͼ��ʾ����(�ں�����)��װ��ʵ��װ�ã�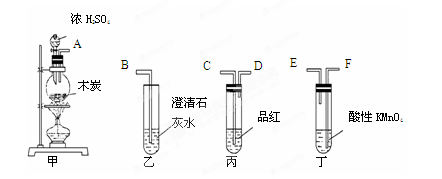
��1���簴������������������������װ�õ���ȷ˳����(����ӿ���ĸ)��
�� �� �� �� �� ��
��2�������ҡ���Ӧ��������ʵ������ű����Ѽ����CO2?
���� ____ ������ ______ ��
��3����������KMnO4��Һ�������� ��
��4��д�����з�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��15�֣�
���Ȼ��ף�PCl3����һ����Ҫ���л��ϳɴ�����ʵ���ҳ��ú���������Cl2��ȡPCl3��װ������ͼ��ʾ��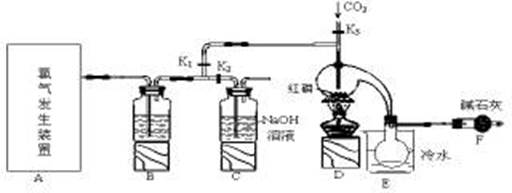
��֪������������Cl2��Ӧ����PCl3�������Cl2��Ӧ����PCl5��PCl3��O2������POCl3(��������)�� POCl3����PCl3��PCl3��ˮ��ǿ��ˮ������H3PO3��HCl��PCl3��POCl3���۷е���±���
| ���� | �۵�/�� | �е�/�� |
| PCl3 | -112 | 75.5 |
| POCl3 | 2 | 105.3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��֪��ICl���۵�Ϊ13��90C���е�Ϊ97��40C����ˮ�⣬���ܷ�����Ӧ��ICl(l��+ Cl2(g��=ICl3(l)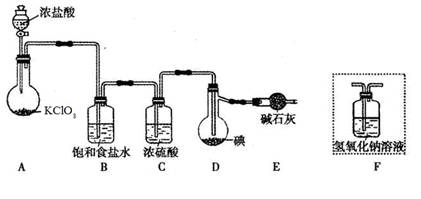
��1��װ��A�з�����Ӧ�Ļ�ѧ����ʽ��____________ ��
��2��װ��B��������______��������װ��F����װ��E������____________ ��
��3�����Ƶõ�ICl����������ICl3���ʣ��ᴿ�ķ�����______ (���ţ���
| A������ | B�������ᾧ | C������ | D����Һ |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ͼ���о�ͭ��Ũ����ķ�Ӧװ�ã�

��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ ��
��3��C�Թܿڽ���NaOH��Һ������������ ��
��4���罫B�Թܻ���D�Թܣ�����ֱ����������BaCl2��Һ��ͨ����һ�����壬������ɫ����������������� �� ����Ҫ����һ�ֻ������һ�ֵ��ʵĻ�ѧʽ��������Ҫ���ɼ�װ������װ�á���
��5��ʵ�������֤��A�Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��6����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
����
| ����1 |  ����ͭ��Ũ���ᷴӦ������ɫ���ʵ�������� |
| ����2 | X���߾������������ͭ��Ũ���ᷴӦ���ɵĺ�ɫ����ΪCu2S��CuS��Cu7S4�е�һ�ֻ��֡� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com