
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1 mol H2SO4��NaOH��Һǡ����ȫ��Ӧ��������ʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽ_____________________��
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ ��
(3)��֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol
��C(s)��O2(g)===CO2(g) �� ��H2����393.5 kJ/mol
��H2(g)��O2(g)===H2O(l ) ��H3����285.8 kJ/mol
д����C(s)��H2(g)��O2(g)��������CH3COOH(l)���Ȼ�ѧ����ʽ ��
(1)H2SO4(aq)��NaOH(aq)===Na2SO4(aq)��H2O(l)�� ��H����57.3 kJ/mol(��ѧ����������H�ɳɱ����仯)
(2)C4H10(g)��O2(g)===4CO2(g)��5H2O(l) ��H����16Q kJ/mol
(3)2C(s)��2H2(g)��O2(g)===CH3COOH(l) ��H����488.3 kJ/mol
�������������Ȼ�ѧ����ʽ����д��
��1���кͷ�Ӧ�Ƿ��ȷ�Ӧ����HС��0����ӦʽΪH2SO4(aq)��NaOH(aq)===Na2SO4(aq)��H2O(l)�� ��H����57.3 kJ/mol��
��2��25 g��ɫ������̼��ƣ������ʵ�����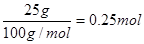 ������̼ԭ���غ��֪�����ɵ�CO2��0.25mol��������1molCO2�ų���������4QkJ��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Զ���ȼ���ȵ��Ȼ�ѧ����ʽΪC4H10(g)��O2(g)===4CO2(g)��5H2O(l) ��H����16Q kJ/mol��
������̼ԭ���غ��֪�����ɵ�CO2��0.25mol��������1molCO2�ų���������4QkJ��ȼ������ָ1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų������������Զ���ȼ���ȵ��Ȼ�ѧ����ʽΪC4H10(g)��O2(g)===4CO2(g)��5H2O(l) ��H����16Q kJ/mol��
��3�������˹���ɵ�Ӧ�úͷ�Ӧ�ȵļ��㡣���ڡ�2���ۡ�2���٣����õ�2C(s)��2H2(g)��O2(g)===CH3COOH(l)�����Է�Ӧ���ǣ�393.5kJ/mol��2��285.8 kJ/mol��2��870.3 kJ/mol����488.3 kJ/mol��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1mol H2SO4��NaOH��Һǡ����ȫ��Ӧ��������ʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽ_____________________��
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ ��
(3)��֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol
��C(s)��O2(g)===CO2(g) �� ��H2����393.5 kJ/mol
��H2(g)��1/2O2(g)===H2O(l) ��H3����285.8 kJ/mol
д����C(s)��H2(g)��O2(g)��������CH3COOH(l)���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1mol H2SO4��NaOH��Һǡ����ȫ��Ӧ��������ʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽ_____________________��
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ ��
(3)��֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol
��C(s)��O2(g)===CO2(g) �� ��H2����393.5 kJ/mol
��H2(g)��1/2O2(g)===H2O(l) ��H3����285.8 kJ/mol
д����C(s)��H2(g)��O2(g)��������CH3COOH(l)���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1mol H2SO4��NaOH��Һǡ����ȫ��Ӧ��������ʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���Ȼ�ѧ����ʽ_____________________��
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ ��
(3)��֪�����Ȼ�ѧ����ʽ��
��CH3COOH(l)��2O2(g)===2CO2(g)��2H2O(l) ��H1����870.3 kJ/mol
��C(s)��O2(g)===CO2(g) �� ��H2����393.5 kJ/mol
��H2(g)��1/2O2(g)===H2O(l) ��H3����285.8 kJ/mol
д����C(s)��H2(g)��O2(g)��������CH3COOH(l)���Ȼ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ���ݽ��Ÿ���ѧ����12���¿���ѧ�Ծ� ���ͣ������
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1 mol H2SO4��NaOH��Һǡ����ȫ��Ӧʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽ_______________ ___
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ_________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ����12���¿���ѧ�Ծ� ���ͣ������
(6��)��Ҫ��д�Ȼ�ѧ����ʽ��
(1)��֪ϡ��Һ�У�1 mol H2SO4��NaOH��Һǡ����ȫ��Ӧʱ���ų�114.6 kJ������д����ʾH2SO4��NaOH��Ӧ���к��ȵ��Ȼ�ѧ����ʽ_______________ ___
(2)25�桢101 kPa�����³��ȼ��һ�����Ķ�������ų�����ΪQ kJ�����ⶨ�������ɵ�CO2ͨ����������ʯ��ˮ�в���25 g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ_________________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com