
����Ŀ��NaI�����Ʊ������л��⻯���ԭ�ϣ�Ҳ����ҽҩ������ȣ���ҵ���õ⡢�������ƺ���мΪԭ�Ͽ�����NaI����������������ͼ��

��1����Ԫ�������ڱ��е�λ��Ϊ______________________��
��2����Ӧ�������ӷ���ʽΪ___________________________________________��
��3����Ӧ�����������м��Ŀ����_________________���������ù���1�г�ʣ����м�⣬���к��ɫ���壬�������мʱ������Ӧ�Ļ�ѧ����ʽΪ____________________________________��
��4����Һ2�г�����H+�⣬һ�������е���������_______________�������ʵ����֤��Һ2�иý��������ӵĴ��ڣ�___________________________________________��
��5����Һ2��һϵ��ת�����Եõ���������������FeC2O4��2H2O����Է�������180������ȡ3.60 g�����������壬�����ط���������ȷֽ���������������)���õ�ʣ�������������¶ȱ仯����������ͼ��ʾ��

������ͼ�����ݣ�������Ϣд������I�����Ļ�ѧ����ʽ��_________________________________��
��300��ʱʣ�����ֻ��һ�ֳɷ��������������д������II�����Ļ�ѧ����ʽ�� ________________��
���𰸡� �������ڵ�VIIA�� 3I2+6OH-![]() 5I-+IO3-+3H2O ��NaIO3��ȫת��ΪNaI 2Fe+3H2O+ NaIO3=NaI+2Fe(OH)3�� Fe2+ ȡ������Һ2�������Ը��������Һ������Һ��ɫ����Fe2+����ȡ������Һ2����K3[Fe(CN)6]��Һ��������ɫ�������ɣ���Fe2+ FeC2O4��2H2O
5I-+IO3-+3H2O ��NaIO3��ȫת��ΪNaI 2Fe+3H2O+ NaIO3=NaI+2Fe(OH)3�� Fe2+ ȡ������Һ2�������Ը��������Һ������Һ��ɫ����Fe2+����ȡ������Һ2����K3[Fe(CN)6]��Һ��������ɫ�������ɣ���Fe2+ FeC2O4��2H2O![]() FeC2O4+2H2O FeC2O4��2H2O
FeC2O4+2H2O FeC2O4��2H2O![]() FeO+CO2��+CO����2H2O
FeO+CO2��+CO����2H2O
����������������������̿�֪���ڼ��������µ�������������Һ��Ӧ�����˵⻯�ƺ͵����ƵĻ��Һ���������۰ѵ����ƻ�ԭΪ�⻯�ƣ����˺���Һ����Ũ�������½ᾧ�����˵õ���Ʒ������1������������ʣ������ۣ�����������ϡ�����ܽ��������������Һ�����������Ա����ۻ�ԭΪ����������
��1����Ԫ�������ڱ��е�λ��Ϊ�������ڵ�VIIA�塣
��2����Ӧ�ٵ����ӷ���ʽΪ3I2+6OH-![]() 5I-+IO3-+3H2O��
5I-+IO3-+3H2O��
��3����Ӧ�ڼ��������м��Ŀ���ǽ�NaIO3��ȫת��ΪNaI���������ù���1�г�ʣ����м�⣬���к��ɫ���壬�ú��ɫ�����������������������мʱ������Ӧ�Ļ�ѧ����ʽΪ2Fe+3H2O+ NaIO3=NaI+2Fe(OH)3����
��4����Һ2�г�����H+�⣬һ�������е���������Fe2+����ΪFe3+�������Ա�H+ǿ��������ʣ�������Ի����ܺ���Fe3+�������ʵ����֤��Һ2��Fe2+�Ĵ���ʱ��Ҫ����Fe3+�ĸ��ţ����Կ���ȡ������Һ2�������Ը��������Һ������Һ��ɫ����Fe2+����ȡ������Һ2����K3[Fe(CN)6]��Һ��������ɫ�������ɣ���Fe2+��
��5����������Ϣ��֪��3.60 g����������������ʵ���Ϊ0.02mol����������������n(Fe)=0.02mol����Ԫ������Ϊ56g/mol��0.02mol=1.12g���ᾧˮ�����ʵ���Ϊ0.04mol���ᾧˮ������Ϊ0.04mol![]() g/mol=0.72g��
g/mol=0.72g��
�ٷ���ͼ�����ݣ�����I����������Ϊ2.88g��3.60g-2.88g=0.72g���������������ʧȥ��ȫ���ᾧˮ�����Թ���I������Ӧ�Ļ�ѧ����ʽΪFeC2O4��2H2O![]() FeC2O4 +2H2O ��
FeC2O4 +2H2O ��
��300��ʱʣ�����ֻ��һ�ֳɷ����������������ͼ��֪������Ϊ1.44g��������Ԫ�ص�����Ϊ1.12g��������Ԫ�ص�����Ϊ1.44g-1.12g=0.32g�����n(O)=0.02mol��n(Fe): n(O)=1:1�����Ը�������ΪFeO�����������غ㶨�ɿ���д������II������Ӧ�Ļ�ѧ����ʽ��FeC2O4��2H2O![]() FeO+CO2��+CO����2H2O ��
FeO+CO2��+CO����2H2O ��


 ��ĩ��ϰ���ϵ�д�
��ĩ��ϰ���ϵ�д� ����ѧ�䵥Ԫ������ĩר����100��ϵ�д�
����ѧ�䵥Ԫ������ĩר����100��ϵ�д� �Ƹ�360�ȶ����ܾ�ϵ�д�
�Ƹ�360�ȶ����ܾ�ϵ�д� ���⿼����Ԫ���Ծ�ϵ�д�
���⿼����Ԫ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����373kʱ����11.5gN2O4����ͨ�����Ϊ500mL������ܱ������У��������ֺ���ɫ��NO2���壬��Ӧԭ��ΪN2O4![]() 2NO2������Ӧ���е�2sʱ��NO2����Ϊ0.01mol����Ӧ����60sʱ�ﵽƽ�⣬��ʱ�����ڻ�������ܶ��������ܶȵ�28.75������ͨ��������գ�
2NO2������Ӧ���е�2sʱ��NO2����Ϊ0.01mol����Ӧ����60sʱ�ﵽƽ�⣬��ʱ�����ڻ�������ܶ��������ܶȵ�28.75������ͨ��������գ�
��1����ʼ2s�ڣ���N2O4��ʾ�ķ�Ӧ����Ϊ___mol��L��1��s��1��
��2���ﵽƽ��ʱ����ϵ��ѹǿ�ǿ�ʼʱ��____����
��3��ƽ��ʱ����_______mol N2O4��
��4��ƽ�����ѹ��������������ٴﵽƽ���NO2��Ũ�Ƚ�_______(������������������������������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�飬���˵����ȷ����

A. ͼ�ף�����NH4Cl������Һ�Ʊ�NH4Cl����
B. ͼ�ң��γ������ĺ�ɫ��Ȫ��֤��HC1��������ˮ
C. ͼ��������һ�����ʵ���Ũ�ȵ�NaOH��Һ������ʱ��ͼ������NaOH��ҺŨ��ƫ��
D. ͼ������ʾװ�����ڳ�ȥ̼�����ƹ����е�����̼����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����й���ʯ�͵�˵����ȷ����(����)
A. ʯ�����ڿ�����������Դ B. ʯ����Ҫ����̼��������Ԫ��
C. ʯ�͵��ѻ��������仯 D. ʯ�ͷ���ĸ���־��Ǵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��������CO��SO2�̵�����Ⱦ��һ�ַ����ǽ����ڴ�����������ת��Ϊ����S����֪��
��CO(g)��0.5O2(g)��CO2(g) ��H����283.0 kJ��mol��1
��S(s)��O2(g)��SO2(g)�� ��H����296.0 kJ��mol��1
����CO��SO2�ķ������Ȼ�ѧ����ʽ��________________________________��
��2��������������ɹ⻯ѧ�����ͳ�������ĵ���Ҫ���塣��֪��
CO(g)��NO2(g)��NO(g)��CO2(g) ��H����a kJ��mol��1(a��0)
2CO(g)��2NO(g)��N2(g)��2CO2(g) ��H����b kJ��mol��1(b��0)
���ñ�״����3.36 L CO��NO2��ԭ��N2(CO��ȫ��Ӧ)��������������ת�Ƶ��ӵ����ʵ���Ϊ______mol���ų�������Ϊ_____(�ú���a��b�Ĵ���ʽ��ʾ)kJ��
��3����CH4����ԭNOxҲ�������������������Ⱦ��������
CH4(g)��4NO2(g)��4NO(g)��CO2(g)��2H2O(g) ��H1����574 kJ��mol��1�� ��
CH4(g)��4NO(g)��2N2(g)��CO2(g)��2H2O(g) ��H2�� ��
��1 mol CH4��ԭNO2��N2�����������зų�������Ϊ867 kJ������H2��_______��
��4����֪�����Ȼ�ѧ����ʽ��
��![]() ��H����285.8kJ/mol
��H����285.8kJ/mol
��![]() ��H����241.8kJ/mol
��H����241.8kJ/mol
��H2��ȼ���ȣ���H��Ϊ________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ���������У�A��B��C��D��Ϊʯī�缫�����������й���0.02mol����ͨ����������������ȷ���ǣ� ��
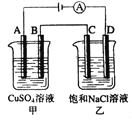
A. ���ձ���A������������ͭ0.64g
B. ���ձ���B���ϵ缫��Ӧʽ4OH����4e���� 2H2O+O2��
C. ���ձ��е����̪��Һ��D�������ȱ��
D. �ձ���C���ϵ缫��ӦʽΪ4H+ + 4e����2H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�˵������ȷ����
A��ij�¶�ʱ�Ļ����Һ��c(H+)=![]() mol��L-1��˵������Һ������(KwΪ���¶�ʱˮ�����ӻ�����)
mol��L-1��˵������Һ������(KwΪ���¶�ʱˮ�����ӻ�����)
B����ˮ�������c(H+)=10-12mol��L-1����Һ��:Na+��Ba2+��HCO3-��Cl-���Դ�������
C����֪Ksp(AgCl)=1.56��10-10�� Ksp(Ag2CrO4)=9.0��10-12������Cl-��CrO42-��Ũ�Ⱦ�Ϊ0.010 mol��L-1��Һ����μ���0.010 mol��L-1��AgNO3��Һʱ��CrO42-�Ȳ�������
D��������pH=7��CH3COOH��NaOH�����Һ����c(Na+)>c(CH3COO-)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����״�����Т�6.72L���顡��3.01��1023���Ȼ�����ӡ���13.6g�����0.2mol NH3 �� ���ж�����������Ĺ�ϵ��С�����ʾ����ȷ���ǣ� ��
A.������ܣ��٣��ڣ���
B.�ܶȣ��٣��ܣ��ۣ���
C.�������ܣ��٣��ۣ���
D.��ԭ�������ڣ��ܣ��ۣ���
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com