
| |||||||||||||||||||
 ��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
��ʦ����ɳ���ʱͬ��ѧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ����һ��2012�������ѧһ�ָ���ѵ������10�µ�2�����ʵķ�����ᴿ(³�ư�) ³�ư� ���ͣ�058
ʵ���ҿ���NH4HCO3��NaClΪԭ���Ʊ����������±����ݺ�ʵ�鲽�裬�ش��������⣺
�Ʊ�����IJ������£�
������50 mLԼ25���Ĵ�ʳ��ˮ��Һ(�ܶȽ���Ϊ1 g/cm3)��
�ھ���ʳ��ˮ(��ʳ���к�������Ca2+��Mg2+��SO![]() ������)�����Ὣ��Һ��pHֵ����7��
������)�����Ὣ��Һ��pHֵ����7��
�ۼ���ʳ��ˮ������Һ�¶ȿ�����30�桫35�森
���ڲ��Ͻ����£��ִν���ϸ��NH4HCO3����ʳ��ˮ�У������Ϻ������¡������Сʱ��
�ݾ��ã����ȹ��ˣ�����ĸҺ������������ˮϴ�����Σ�
���������գ��õ����
(1)������У�������Һ�����һ��������________��
(2)������У�SO![]() �Ƿ���Ҫ��ȥ��________��������________��
�Ƿ���Ҫ��ȥ��________��������________��
(3)����������в����Ĺ�ͬĿ����________��
(4)������о����ϴ��Һ����Ҫ���е�������������________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ�������и߶���ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��8�֣�ʵ���ҿ���NH4HCO3��NaClΪԭ���Ʊ����������±����ݺ�ʵ�鲽�裬�ش��������⣺ 30��ʱ�����ε��ܽ�ȣ�g
| NaCl | NH4HCO3 | NaHCO3 | NH4Cl | Na2SO4 | ��NH4��2SO4 | CaCl2 | MgCl2 | CaSO4 |
| 36.3 | 27.0 | 11.1 | 41.4 | 40.8 | 78 | 51.7 | 26.2 | 0.165 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���˽̰���л�ѧѡ��6 2.1 ���ʵķ�����ᴿ��ϰ���������棩 ���ͣ�ʵ����
ʵ���ҿ���NH4HCO3��NaClΪԭ���Ʊ����������±����ݺ�ʵ�鲽�裬�ش��������⣺ 30��ʱ�����ε��ܽ�ȣ�g
|
NaCl |
NH4HCO3 |
NaHCO3 |
NH4Cl |
Na2SO4 |
|
CaCl2 |
MgCl2 |
CaSO4 |
|
36.3 |
27.0 |
11.1 |
41.4 |
40.8 |
78 |
51.7 |
26.2 |
0.165 |
�Ʊ�����IJ������£�
�� ����50 mLԼ25���Ĵ�ʳ��ˮ��Һ���ܶȽ���Ϊ1 g��cm3����
�� ����ʳ��ˮ����ʳ���к�������Ca2+��Mg2+��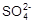 �����ʣ������Ὣ��Һ��pHֵ����7��
�����ʣ������Ὣ��Һ��pHֵ����7��
�� ����ʳ��ˮ������Һ�¶ȿ�����30 �� 35�档
�� �ڲ��Ͻ����£��ִν���ϸ��NH4HCO3����ʳ��ˮ�У������Ϻ������¡� �����Сʱ��
�� ���ã����ȹ��ˣ�����ĸҺ������������ˮϴ�����Ρ�
�� ���������գ��õ����
��1��������У�������Һ�����һ��������______________________��
��2��������У�SO42-�Ƿ���Ҫ��ȥ��_________��������________________
��3������������в����Ĺ�ͬĿ����_____________��
��4��������о����ϴ��Һ����Ҫ���е�������������____________����ͨ������£��������������ӵķ���_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���㽭ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��8�֣�ʵ���ҿ���NH4HCO3��NaClΪԭ���Ʊ����������±����ݺ�ʵ�鲽�裬�ش��������⣺ 30��ʱ�����ε��ܽ�ȣ�g
|
NaCl |
NH4HCO3 |
NaHCO3 |
NH4Cl |
Na2SO4 |
��NH4��2SO4 |
CaCl2 |
MgCl2 |
CaSO4 |
|
36.3 |
27.0 |
11.1 |
41.4 |
40.8 |
78 |
51.7 |
26.2 |
0.165 |
�Ʊ�����IJ������£�
�� ����50 mLԼ25���Ĵ�ʳ��ˮ��Һ���ܶȽ���Ϊ1 g��mL����
�� ����ʳ��ˮ����ʳ���к�������Ca2+��Mg2+��SO42-�����ʣ��������Ὣ��Һ��pHֵ����7��
�� ����ʳ��ˮ������Һ�¶ȿ�����30 �� 35�档
�� �ڲ��Ͻ����£��ִν���ϸ��NH4HCO3����ʳ��ˮ�У������Ϻ������¡� �����Сʱ��
�� ���ã����ȹ��ˣ�����ĸҺ������������ˮϴ�����Ρ�
�� ���������գ��õ����
��1��������У�������Һ�����һ�������� ��
��2��������У�SO42-�Ƿ���Ҫ����ij���Լ���ȥ�� ��������
��3������������в����Ĺ�ͬĿ���� ��
��4��������о����ϴ��Һ����Ҫ���е������������� ����ͨ������£��������������ӵ�ʵ�����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com