
 ��ͼ�Ǽ״�--������ع���ʱ��ʾ��ͼ���״��ڴ����������ṩ���ӣ�H+���͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������˵������ȷ���ǣ�������
��ͼ�Ǽ״�--������ع���ʱ��ʾ��ͼ���״��ڴ����������ṩ���ӣ�H+���͵��ӣ����Ӿ����·�����Ӿ��ڵ�·������һ����������Ӧ������˵������ȷ���ǣ�������| 2 |
| 3 |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��������������2010��8��19���ڶ�����ζ���ѡ��ݳԹ�������6���ˣ������ȴ����ɵ���ʡ���ҽԺ�IJ����ϣ���Ͼ�Ϊ�״��ж����״���һ����ɫ��������ȼ���ӷ����ж�Һ�壬���оƾ���ζ������5��10������˫Ŀʧ�����������ûᵼ��������
��������������2010��8��19���ڶ�����ζ���ѡ��ݳԹ�������6���ˣ������ȴ����ɵ���ʡ���ҽԺ�IJ����ϣ���Ͼ�Ϊ�״��ж����״���һ����ɫ��������ȼ���ӷ����ж�Һ�壬���оƾ���ζ������5��10������˫Ŀʧ�����������ûᵼ��������| 1 |
| 2 |
| 3 |
| 2 |
| 3 |
| 2 |
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1����������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��CH2O����������Ҫ������Ⱦ��֮һ����е���-19.5�棩���״��ǡ��پơ��е���Ҫ�к����ʣ���е���64.65�棩���״��ķе����Ը��ڼ�ȩ����Ҫԭ����
��1����������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��CH2O����������Ҫ������Ⱦ��֮һ����е���-19.5�棩���״��ǡ��پơ��е���Ҫ�к����ʣ���е���64.65�棩���״��ķе����Ը��ڼ�ȩ����Ҫԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
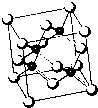 ��1����������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��������Ҫ������Ⱦ��֮һ����е���-19.5
��1����������������������ߣ���������Ļ�����ȫΪ���������ӣ����ڵĻ�����ȫ��ʳƷ��ȫҲԽ��ԽΪ��������ע����ȩ��������Ҫ������Ⱦ��֮һ����е���-19.5�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧ������У��Ͼ���ѧģ���Ծ���һ���������棩 ���ͣ������
 2O2��g��=CO2��g��+2H2��g������H=-192.9kJ?mol-1
2O2��g��=CO2��g��+2H2��g������H=-192.9kJ?mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ�������и߿���ѧһģ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com