
����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���һ����̼�������Ļ����������ú̿��ˮ(����)��Ӧ�Ƶã����ֳ�ˮú����
(1)��д����ȡˮú������Ҫ��ѧ����ʽ______________________��
(2)Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ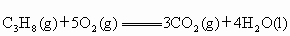 ��
�� ��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ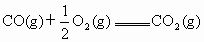 ��
��
 �ԱȽ�ͬ���ʵ�����
�ԱȽ�ͬ���ʵ�����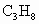 ��COȼ�գ�������������ֵԼΪ______________��
��COȼ�գ�������������ֵԼΪ______________��
(3)��֪����ȼ�յ��Ȼ�ѧ����ʽΪ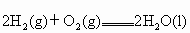 ��
��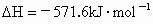 ���ԱȽ�ͬ�����������ͱ���ȼ�գ�������������ֵԼΪ______________��
���ԱȽ�ͬ�����������ͱ���ȼ�գ�������������ֵԼΪ______________��
(4)������δ������Դ����������������֮�⣬�����е��ŵ���_________��
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��8�֣�����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g)== 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ��![]() ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ��![]() ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)+ O2(g) == 2H2O(l)����H=�C571.6 kJ�� ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��Ϫ�ظ��и߶����ڲ��νο��Ի�ѧ���� ���ͣ������
��8�֣�����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g) == 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ�� ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ�� ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g) + O2(g) == 2H2O(l)����H=�C571.6 kJ�� ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ�߶����ڲ��νο��Ի�ѧ���� ���ͣ������
��8�֣�����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g)
== 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ�� ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ�� ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g)
+ O2(g) == 2H2O(l)����H=�C571.6 kJ�� ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ��ж���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʹ�õ�ȼ�ϣ��ִ����ú����Һ��ʯ������ú������Ҫ�ɷ���CO��H2�Ļ�����壬����ú̿��ˮ������Ӧ�Ƶã����ֳ�ˮú����
��1����д����ȡˮú������Ҫ��ѧ����ʽ____________________________.
��2��Һ��ʯ��������Ҫ�ɷ��DZ��飬����ȼ�յ��Ȼ�ѧ����ʽΪ
C3H8(g) +5O2(g) == 3CO2(g) + 4H2O(l)����H=�C2220.0 kJ��![]() ,
,
��֪CO����ȼ�յ��Ȼ�ѧ����ʽΪ��
CO(g) +1/2O2(g) == CO2(g) ����H=�C282.57kJ��![]() ,
,
�ԱȽ�ͬ������C3H8��COȼ�գ�������������ֵԼΪ_________��1��
��3����֪����ȼ�յ��Ȼ�ѧ����ʽΪ2H2(g) + O2(g) == 2H2O(l)����H=�C571.6 kJ��![]() ,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
,�ԱȽ�ͬ������H2��C3H8ȼ�գ�������������ֵԼΪ____________��1��
��4��������δ������Դ������Դ�ḻ֮�⣬�����е��ŵ���____________________
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com