

 ��
������ A�IJ���������������һ�����ҵ�ʯ�ͻ���ˮƽ��D�Ǿ��й�����ζ��������������ͼ��ת����֪��AΪCH2=CH2��A��ˮ��Ӧ����BΪCH3CH2OH��B�����������������CΪCH3COOH��B��C����������Ӧ����DΪCH3COOCH2CH3��
��1�����͡�ú�͡�ʯ���͵ȳɷݾ�ΪҺ̬������
��2��A�к�̼̼˫����C�к�-COOH��
��3��A��B��̼̼˫��ת��Ϊ������B+C��DΪ������Ӧ��
��4��B��Cu�����ȵ���������������Ӧ��������ȩ��
��5��������̼���Ʒ�Ӧ�������������ֲ㣻
��6���ٶ��麬4��Cԭ�ӣ�ͬ���칹���������4����3��C��
�������һ��ͬ���칹�壬����һ��ȡ������4�֣�������к�4��H��
��� �⣺��1�����͡�ú�͡�ʯ���͵ȳɷݾ�ΪҺ̬����������ʯ�ͻ�����͡�ú�͡�ʯ���͵ȳɷݵķ����Ƿ��������ʴ�Ϊ����������
��2��A��C�к��еĹ����ŷֱ���̼̼˫�����Ȼ����ʴ�Ϊ��̼̼˫�����Ȼ���
��3��A��B��̼̼˫��ת��Ϊ��������Ӧ����Ϊ�ӳɷ�Ӧ��B+C��DΪ������Ӧ����ӦΪCH3COOH+CH3CH2OH $?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
�ʴ�Ϊ���ӳɷ�Ӧ��CH3COOH+CH3CH2OH $?_{��}^{ŨH_{2}SO_{4}}$CH3COOCH2CH3+H2O��
��4��B��Cu�����ȵ���������������Ӧ��������ȩ����ӦΪ2CH3CH2OH+O2$��_{��}^{Cu}$ 2CH3CHO+2H2O��
�ʴ�Ϊ��2CH3CH2OH+O2$��_{��}^{Cu}$ 2CH3CHO+2H2O��
��5��������ˮ���Ҵ������²���Ӧ������������NaOH��Ӧ����������̼���Ʒ�Ӧ�������������ֲ㣬��Ҫ��ȥ����Ӧѡ�õ��Լ�ΪB��
�ʴ�Ϊ��B��
��6���ٶ��麬4��Cԭ�ӣ�ͬ���칹���������4����3��C��ͬ���칹��Ľṹ��ʽΪCH3CH2CH2CH3�� ��
��
�ʴ�Ϊ��CH3CH2CH2CH3�� ��
��
�������һ��ͬ���칹�壬����һ��ȡ������4�֣�������к�4��H������ͬ���칹��Ľṹ��ʽΪ��CH3��2CHCH2CH3��
�ʴ�Ϊ����CH3��2CHCH2CH3��
���� ���⿼���л�����ƶϣ�Ϊ��Ƶ���㣬�����л�������ʡ��л���Ӧ�ƶ��л���Ϊ���Ĺؼ������ط������ƶ������Ŀ��飬ע�ⳣ���л�������ʼ�Ӧ�ã���Ŀ�ѶȲ���


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
 þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ������ ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������
þ�����ֱ����Ũ�ȡ�������Ĺ���ϡ���ᷴӦ������ ����������V����ʱ�䣨t����ϵ��ͼ����Ӧ��þ�����ģ�������| A�� | Ħ������֮��Ϊ 2��3 | B�� | ���ʵ���֮��Ϊ 3��2 | ||
| C�� | ����֮��Ϊ 3��2 | D�� | ��Ӧ����֮��Ϊ 3��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
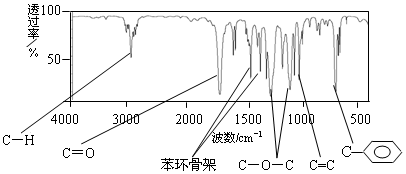
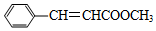 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 �� D�����й����ŵ��������Ȼ���
�� D�����й����ŵ��������Ȼ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ڰ��ף�P4�������У�Pԭ����P-P����Ŀ��Ϊ2��3 | |
| B�� | ���Ӿ����ж����ڷ��»����������ڶ����ڹ��ۼ� | |
| C�� | HF��HCl��HBr��HI�������ʵķе��������� | |
| D�� | �ɱ����Ȼ�立ֱ����ȱ�Ϊ�������˷������Ӽ����������ͬ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | Ŀ�� | ���뷽�� | ԭ�� |
| A | ��������ˮ�ĵ� | ����ȡ | �����ܶȱ�ˮС |
| B | ���������������Ҵ� | ��Һ | �����������Ҵ����ܶȲ�ͬ |
| C | ��ȥKNO3�����л��ӵ�NaCl | �ؽᾧ | KNO3��ˮ�е��ܽ�Ⱥܴ� |
| D | ��ȥ�����е�̼����� | ���� | ���ȶ���ͬ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| �ɷ� | ������mg/L�� | �ɷ� | ������mg/L�� |
| Na+ | 10560 | Cl- | 18980 |
| Mg2+ | 1272 | Br- | 64 |
| Ca2+ | 400 | SO42- | 2560 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Ũ������������Ӧ��ֻ��������� | |
| B�� | ������Ӧ�����ȵ� | |
| C�� | ������Ӧ�Ļ�������ȥ�ǻ�����ȥ�ǻ��ϵ���ԭ�� | |
| D�� | ������ӦҲ����ȡ����Ӧ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com