
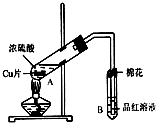
 ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���
ij�о�С������ͼ��ʾװ�ý���ͭ��Ũ���ᷴӦ��ʵ���о���| �������� | �����뾧��������� | ���Ⱥ���������������� |
| 11.7g | 22.7g | 18.9g |
���� ��1�������������Ư���ԣ���ʹƷ����Һ��ɫ��
��2�����������£�Cu��Ũ���ᷴӦ����CuSO4��SO2��H2O��
��3���������������������������ж�������ֱ���ſգ������ü�Һ���գ�
��4�����������£�˫��ˮ������Cu����ͭ���ӣ����Ρ����ᶼ������Cu��
��5���ᾧˮ����=��22.7-18.9��g=3.8g������ͭ����=��18.9g-11.7g��=7.2g��n��H2O����n��CuSO4��=$\frac{3.8g}{18g/mol}$��$\frac{7.2g}{160g/mol}$��4.7��5����ᾧˮ������ƫС��
A������ͭ�����к��в��ӷ������ʵ��½ᾧˮ����ƫС��
B��ʵ��ǰ���������ʪ��ˮ���½ᾧˮ����ƫ��
C������ʱ�о���ɽ���ȥ��Ӱ��ᾧˮ������
D������ʧˮ��¶���ڿ�������ȴ���½ᾧˮ����ƫС��
��� �⣺��1�������������Ư���ԣ���ʹƷ����Һ��ɫ�����Կ�����������Ʒ����Һ��ɫ���ʴ�Ϊ��Ʒ����Һ��ɫ��
��2�����������£�Cu��Ũ���ᷴӦ����CuSO4��SO2��H2O����Ӧ����ʽΪCu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
�ʴ�Ϊ��Cu+2H2SO4��Ũ��$\frac{\underline{\;\;��\;\;}}{\;}$CuSO4+SO2��+2H2O��
��3���������������������������ж�������ֱ���ſգ������ü�Һ���գ�������պȡ����ҺΪ��Һ����NaOH��Һ���ʴ�Ϊ��NaOH��Һ��
��4�����������£�˫��ˮ������Cu����ͭ���ӣ����ӷ���ʽΪCu+H2O2+2H+=Cu2++2H2O�����Ρ����ᶼ������Cu����Ӧ����Һ����ϡ����ʣ�࣬���Կ��Լ����������������ƣ��������ܽ����������������������������������������������Cu����ͭ�Σ�
�ʴ�Ϊ��Cu+H2O2+2H+=Cu2++2H2O��Fe2O3��NaNO3��
��5���ᾧˮ����=��22.7-18.9��g=3.8g������ͭ����=��18.9g-11.7g��=7.2g��n��H2O����n��CuSO4��=$\frac{3.8g}{18g/mol}$��$\frac{7.2g}{160g/mol}$��4.7��5����ᾧˮ������ƫС��
A������ͭ�����к��в��ӷ������ʵ��½ᾧˮ����ƫС������ȷ��
B��ʵ��ǰ���������ʪ��ˮ���½ᾧˮ����ƫ�ʴ���
C������ʱ�о���ɽ���ȥ����Ϊ��������ɷֺ;���ɷ���ͬ�����Բ�Ӱ��ᾧˮ�������ʴ���
D������ʧˮ��¶���ڿ�������ȴ����ˮ����ͭ����ˮ���½ᾧˮ����ƫС������ȷ��
�ʴ�Ϊ��ƫС��AD��
���� ������ͭ��Ũ���ᷴӦΪ���忼������ʵ�鷽����ƣ���ȷʵ��ԭ�������������ǽⱾ��ؼ����ѵ��ǣ�4����������Һ���ܽ�Cu�����ʵ�ѡȡ���״���������������Ŀ�Ѷ��еȣ�


 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ͬ������ͬ�ܶȵ�C2H4��CO | B�� | ͬ�¶�ͬ�����C2H4��NO | ||
| C�� | ͬ�¶�ͬѹǿ��CO��N2 | D�� | ͬ���ͬѹǿ��N2��N2H4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
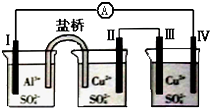
| A�� | �缫�����ܽ� | B�� | �缫����������Ӧ | ||
| C�� | �������缫���A���缫�� | D�� | �缫��ĵ缫��Ӧ��Cu2++2e-=Cu |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | c��CH3COO -����c��CH3COOH�� | B�� | c��CH3COO -����c��Na+����c��H+����c��OH -�� | ||
| C�� | c��Na+��=c��CH3COO -��=0.01mol•L-1 | D�� | c��CH3COOH��+c��CH3COO -��=0.02mol•L-1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 4��ʱ40mlˮ | B�� | 0.8mol���� | ||
| C�� | 9.03��1022�������� | D�� | 54g�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢� | B�� | �٢� | C�� | �ڢۢ� | D�� | �ۢܢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��1mol����������ᷴӦ�����ӷ���ʽ��N2H4+2H+�TN2H62+ ����������ԭ��Ӧ����H2O2���ƣ��ȿ������������ֿ�����ԭ�������ݼ�̬�����������������ԭ����N2H4�е�Ԫ�صĻ��ϼ�Ϊ-2�ۣ��ȿ������ߣ��ֿ��Խ��ͣ�8gҺ̬����Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ��ų�375kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-1 500 kJ/mol��
��1mol����������ᷴӦ�����ӷ���ʽ��N2H4+2H+�TN2H62+ ����������ԭ��Ӧ����H2O2���ƣ��ȿ������������ֿ�����ԭ�������ݼ�̬�����������������ԭ����N2H4�е�Ԫ�صĻ��ϼ�Ϊ-2�ۣ��ȿ������ߣ��ֿ��Խ��ͣ�8gҺ̬����Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ��������̬���ʣ��ų�375kJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2H4��l��+2H2O2��l���TN2��g��+4H2O��g����H=-1 500 kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 0.0025 mol•L-1 | B�� | 0.0001mol•L-1 | C�� | 0.001mol•L-1 | D�� | 0.005mol•L-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com