ÒŃÖȘAĄąBĄąCĄąDĄąEĄąF¶ŒÊÇÖÜÆÚ±íÖĐÇ°ËÄÖÜÆÚ”ÄÔȘËŰŁźËüĂÇ”ÄÔŚÓĐòÊęÒÀŽÎÔöŽóŁźÆäÖĐAĄąCÔŚÓ”ÄLČăÓĐ2žöÎŽłÉ¶Ô”çŚÓŁźDÓëEÍŹÖśŚćŁŹD”ĶțŒÛŃôÀëŚÓÓëC”ÄÒőÀëŚÓŸßÓĐÏàÍŹ”Ä”çŚÓČăœáč裟F
3+ÀëŚÓMČă3dčì”À”çŚÓÎȘ°ëÂúŚŽÌŹŁźÇëžùŸĘÒÔÉÏÇéżöŁŹ»ŰŽđÏÂÁĐÎÊÌâŁșŁšŽđÌâʱŁŹÓĂËù¶ÔÓŠ”ÄÔȘËŰ·ûșƱíÊŸŁ©
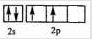
Łš1Ł©ĐŽłöCÔŚÓ”ÄŒÛČă”çŚÓĆĆČŒÍŒ
ŁŹFλÓÚÖÜÆÚ±í
d
d
ÇűŁź
Łš2Ł©AĄąBĄąC”Ä”ÚÒ»”çÀëÄÜÓÉĐĄ”œŽó”ÄËłĐòÎȘ
CŁŒOŁŒN
CŁŒOŁŒN
ŁźŁšĐŽÔȘËŰ·ûșĆŁ©
Łš3Ł©FșÍÖÊŚÓÊęÎȘ25”ÄM”ÄČż·Ö”çÀëÄÜÊęŸĘÁĐÓÚϱí
| ÔȘËŰ |
M |
F |
| ”çÀëÄÜŁškJ?mol-1Ł© |
I1 |
717 |
759 |
| I2 |
1509 |
1561 |
| I3 |
3248 |
2957 |
±ÈœÏÁœÔȘËŰ”ÄI
2ĄąI
3żÉÖȘŁŹÆűÌŹM
2+ÔÙʧȄһžö”çŚÓ±ÈÆűÌŹF
2+ÔÙʧȄһžö”çŚÓÄŃŁź¶ÔŽËŁŹÄă”ÄœâÊÍÊÇ
Mn2+”Ä3dčì”À”çŚÓĆĆČŒÎȘ°ëÂúŚŽÌŹœÏÎȶš
Mn2+”Ä3dčì”À”çŚÓĆĆČŒÎȘ°ëÂúŚŽÌŹœÏÎȶš
Łź
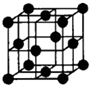
Łš4Ł©Ÿ§°ûÖĐFÔŚÓ”ÄĆäλÊęÎȘ
8
8
ŁŹÈôFÔŚÓ”Ä°ëŸ¶ÎȘrcmŁŹÔòFŸ§Ìć”ÄĂܶÈÎȘ
ŁšÓĂșŹr”ıíŽïÊœ±íÊŸŁ©ŁŹžĂŸ§°ûÖĐÔŚÓżŐŒäÀûÓĂÂÊÎȘ
68%
68%
Łź
Łš5Ł©H
2SșÍCÔȘËŰ”ÄÇ⻯ÎïŁš·ÖŚÓÊœÎȘH
2C
2”ÄÖśÒȘÎïÀíĐÔÖʱȜÏÈçÏÂ
|
ÈÛ”ă/K |
·Đ”ă/K |
±êŚŒŚŽżöʱÔÚËźÖĐ”ÄÈÜœâ¶È |
| H2S |
187 |
202 |
2.6 |
| H2C2 |
272 |
423 |
±ÈÈÎÒâ±È»„ÈÜ |
H
2SșÍH
2C
2”ÄÏà¶Ô·ÖŚÓÖÊÁż»ù±ŸÏàÍŹŁŹÔìłÉÉÏÊöÎïÀíĐÔÖÊČîÒì”ÄÖśÒȘÔÒò
H2O2·ÖŚÓŒäŽæÔÚÇâŒüŁŹÓëËź·ÖŚÓżÉĐÎłÉÇâŒü
H2O2·ÖŚÓŒäŽæÔÚÇâŒüŁŹÓëËź·ÖŚÓżÉĐÎłÉÇâŒü
Łź



 Ąą
Ąą ŁŹÔŚÓĐòÊęAĐĄÓÚCŁŹÔòAÎȘCÔȘËŰŁŹCÎȘOÔȘËŰŁŹÔòBÎȘNÔȘËŰŁŹD”ĶțŒÛŃôÀëŚÓÓëC”ÄÒőÀëŚÓŸßÓĐÏàÍŹ”Ä”çŚÓČăœáč裏DÓŠÎȘMgÔȘËŰŁŹDÓëEÍŹÖśŚćŁŹÔòEÓŠÎȘCaÔȘËŰŁŹF3+ÀëŚÓMČă3dčì”À”çŚÓÎȘ°ëÂúŚŽÌŹŁŹÔòÀëŚÓ”ÄșËÍâ”çŚÓÊęÎȘ23ŁŹF”ÄÔŚÓĐòÊęÎȘ26ŁŹÓŠÎȘFeÔȘËŰŁŹœáșÏÔȘË۶ÔÓŠÔŚÓ”Äœáč襹”„ÖÊŒ°»ŻșÏÎï”ÄĐÔÖÊœâŽđžĂÌ⣟
ŁŹÔŚÓĐòÊęAĐĄÓÚCŁŹÔòAÎȘCÔȘËŰŁŹCÎȘOÔȘËŰŁŹÔòBÎȘNÔȘËŰŁŹD”ĶțŒÛŃôÀëŚÓÓëC”ÄÒőÀëŚÓŸßÓĐÏàÍŹ”Ä”çŚÓČăœáč裏DÓŠÎȘMgÔȘËŰŁŹDÓëEÍŹÖśŚćŁŹÔòEÓŠÎȘCaÔȘËŰŁŹF3+ÀëŚÓMČă3dčì”À”çŚÓÎȘ°ëÂúŚŽÌŹŁŹÔòÀëŚÓ”ÄșËÍâ”çŚÓÊęÎȘ23ŁŹF”ÄÔŚÓĐòÊęÎȘ26ŁŹÓŠÎȘFeÔȘËŰŁŹœáșÏÔȘË۶ÔÓŠÔŚÓ”Äœáč襹”„ÖÊŒ°»ŻșÏÎï”ÄĐÔÖÊœâŽđžĂÌ⣟ Ąą
Ąą ŁŹÔŚÓĐòÊęAĐĄÓÚCŁŹÔòAÎȘCÔȘËŰŁŹCÎȘOÔȘËŰŁŹÔòBÎȘNÔȘËŰŁŹD”ĶțŒÛŃôÀëŚÓÓëC”ÄÒőÀëŚÓŸßÓĐÏàÍŹ”Ä”çŚÓČăœáč裏DÓŠÎȘMgÔȘËŰŁŹDÓëEÍŹÖśŚćŁŹÔòEÓŠÎȘCaÔȘËŰŁŹF3+ÀëŚÓMČă3dčì”À”çŚÓÎȘ°ëÂúŚŽÌŹŁŹÔòÀëŚÓ”ÄșËÍâ”çŚÓÊęÎȘ23ŁŹF”ÄÔŚÓĐòÊęÎȘ26ŁŹÓŠÎȘFeÔȘËŰŁŹÔò
ŁŹÔŚÓĐòÊęAĐĄÓÚCŁŹÔòAÎȘCÔȘËŰŁŹCÎȘOÔȘËŰŁŹÔòBÎȘNÔȘËŰŁŹD”ĶțŒÛŃôÀëŚÓÓëC”ÄÒőÀëŚÓŸßÓĐÏàÍŹ”Ä”çŚÓČăœáč裏DÓŠÎȘMgÔȘËŰŁŹDÓëEÍŹÖśŚćŁŹÔòEÓŠÎȘCaÔȘËŰŁŹF3+ÀëŚÓMČă3dčì”À”çŚÓÎȘ°ëÂúŚŽÌŹŁŹÔòÀëŚÓ”ÄșËÍâ”çŚÓÊęÎȘ23ŁŹF”ÄÔŚÓĐòÊęÎȘ26ŁŹÓŠÎȘFeÔȘËŰŁŹÔò ŁŹFÎȘFeÔȘËŰŁŹÔŚÓșËÍâșŹÓĐd”çŚÓŁŹÎ»ÓÚÖÜÆÚ±ídÇűŁŹ
ŁŹFÎȘFeÔȘËŰŁŹÔŚÓșËÍâșŹÓĐd”çŚÓŁŹÎ»ÓÚÖÜÆÚ±ídÇűŁŹ Ł»dŁ»
Ł»dŁ»




 ÒŃÖȘAĄąBĄąCĄąDĄąEÎćÖÖÎïÖÊÓĐÈçÍŒËùÊŸ”ÄŚȘ»ŻčŰÏ”ŁšČż·Ö·ŽÓŠÎ·ŽÓŠÌőŒțÎŽÁĐłöŁŹÈôœâÌâʱĐèÒȘŁŹżÉŚśșÏÀíŒÙÉèŁ©ŁŹÇÒÎćÖÖÎïÖÊÖĐŸùșŹÓĐAÔȘËŰŁź
ÒŃÖȘAĄąBĄąCĄąDĄąEÎćÖÖÎïÖÊÓĐÈçÍŒËùÊŸ”ÄŚȘ»ŻčŰÏ”ŁšČż·Ö·ŽÓŠÎ·ŽÓŠÌőŒțÎŽÁĐłöŁŹÈôœâÌâʱĐèÒȘŁŹżÉŚśșÏÀíŒÙÉèŁ©ŁŹÇÒÎćÖÖÎïÖÊÖĐŸùșŹÓĐAÔȘËŰŁź ŁšąńŁ©ÍšłŁÇéżöÏÂŁŹÎąÁŁAșÍBÎȘ·ÖŚÓŁŹCșÍEÎȘŃôÀëŚÓŁŹDÎȘÒőÀëŚÓŁŹËüĂǶŒșŹÓĐ10žö”çŚÓŁ»BÈÜÓÚAșóËù”Ă”ÄÎïÖÊżÉ”çÀëłöCșÍDŁ»AĄąBĄąEÈęÖÖÎąÁŁ·ŽÓŠșóżÉ”ĂCșÍÒ»ÖÖ°ŚÉ«łÁ”íŁźÇë»ŰŽđŁș
ŁšąńŁ©ÍšłŁÇéżöÏÂŁŹÎąÁŁAșÍBÎȘ·ÖŚÓŁŹCșÍEÎȘŃôÀëŚÓŁŹDÎȘÒőÀëŚÓŁŹËüĂǶŒșŹÓĐ10žö”çŚÓŁ»BÈÜÓÚAșóËù”Ă”ÄÎïÖÊżÉ”çÀëłöCșÍDŁ»AĄąBĄąEÈęÖÖÎąÁŁ·ŽÓŠșóżÉ”ĂCșÍÒ»ÖÖ°ŚÉ«łÁ”íŁźÇë»ŰŽđŁș