
МзДМЪЧвЛжжгХжЪЕФвКЬхШМСЯЃЌCOКЭCO2ОљПЩгУгкКЯГЩМзДМЁЃ
ФПЧАЙЄвЕЩЯгавЛжжЗНЗЈЪЧгУCO2РДЩњВњШМСЯМзДМЁЃвЛЖЈЬѕМўЯТЗЂЩњЗДгІЃК
CO2(g) +3H2(g) ЃНCH3OH(g)+H2O(g) ЁїH1
ЃЈ1ЃЉвбжЊЃК2CO(g) +O2(g) ЃН2CO2(g) ЁїH2
2H2(g)+O2(g) ЃН2H2O(g) ЁїH3
дђCO(g) + 2H2(g)  CH3OH(g)ЕФЁїHЃН ЁЃ
CH3OH(g)ЕФЁїHЃН ЁЃ
ЃЈ2ЃЉгЩCOКЯГЩМзДМЪБЃЌCOдкВЛЭЌЮТЖШЯТЕФЦНКтзЊЛЏТЪгыбЙЧПЕФЙиЯЕШчЯТЭМЫљЪОЁЃ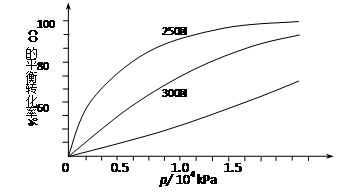
ИУЗДгІІЄH 0ЃЈЬюЁА>ЁБЛђЁА <ЁБЃЉЁЃЪЕМЪЩњВњЬѕМўПижЦдк250ЁцЁЂ1.3ЁС104kPaзѓгвЃЌбЁдёДЫбЙЧПЕФРэгЩЪЧ ЁЃ
ЃЈ3ЃЉ
вЛЖЈЮТЖШЯТЃЌЯђ2LУмБеШнЦїжаМгШы1mol CH3OH (g)ЃЌЗЂЩњЗДгІЃКCH3OH(g)  CO(g) + 2H2(g)ЃЌH2ЮяжЪЕФСПЫцЪБМфЕФБфЛЏШчЭМЫљЪОЁЃ0~2 minФкЕФЦНОљЗДгІЫйТЪv(CH3OH)= ЁЃ
CO(g) + 2H2(g)ЃЌH2ЮяжЪЕФСПЫцЪБМфЕФБфЛЏШчЭМЫљЪОЁЃ0~2 minФкЕФЦНОљЗДгІЫйТЪv(CH3OH)= ЁЃ
ИУЮТЖШЯТЃЌCO(g) + 2H2(g)  CH3OH(g)ЕФЦНКтГЃЪ§K= ЁЃ
CH3OH(g)ЕФЦНКтГЃЪ§K= ЁЃ
ЯрЭЌЮТЖШЯТЃЌШєПЊЪММгШыCH3OH(g)ЕФЮяжЪЕФСПЪЧдРДЕФ2БЖЃЌдђ ЪЧдРДЕФ2БЖЁЃ
aЃЎЦНКтГЃЪ§ bЃЎCH3OHЕФЦНКтХЈЖШ cЃЎДяЕНЦНКтЕФЪБМф dЃЎЦНКтЪБЦјЬхЕФУмЖШ
ЃЈ4ЃЉвдCH3OHЮЊШМСЯЃЈвдKOHШмвКзїЕчНтжЪШмвКЃЉПЩжЦГЩCH3OHШМСЯЕчГиЁЃ
ЂйГфШыCH3OHЕФЕчМЋЮЊ МЋЃЛ
ЂкИКМЋЗДгІЕФЕчМЋЗДгІЪНЮЊ ЁЃ
ЃЈ1ЃЉЁїH1+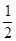 ЁїH2 Ѓ
ЁїH2 Ѓ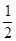 ЁїH3 ЁЃЃЈ2ЗжЃЉ
ЁїH3 ЁЃЃЈ2ЗжЃЉ
ЃЈ2ЃЉ<ЃЛЃЈ1ЗжЃЉ дк1.3ЁС104kPaЯТЃЌCOЕФзЊЛЏТЪвбОКмИпЃЌШчЙћдіМгбЙЧПCOЕФзЊЛЏТЪЬсИпВЛДѓЃЌЖјЩњВњГЩБОдіМгЃЌЕУВЛГЅЪЇЁЃЃЈ3ЗжЃЉ
ЃЈ3ЃЉ0.125molЁЄLЃ1ЁЄ minЃ1ЃЛЃЈ2ЗжЃЉ 4 L2ЁЄmolЃ2ЃЛЃЈ2ЗжЃЉ dЁЃЃЈ1ЗжЃЉ
ЃЈ4ЃЉЂйИКЃЛЃЈ1ЗжЃЉ ЂкCH3OH Ѓ 6eЃ+8OHЃ= CO32Ѓ+6H2OЁЃЃЈ2ЗжЃЉ
НтЮі


 53ЫцЬУВтЯЕСаД№АИ
53ЫцЬУВтЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
МзДМЪЧвЛжжгХжЪЕФвКЬхШМСЯЃЌCOКЭCO2ОљПЩгУгкКЯГЩМзДМЁЃ
ФПЧАЙЄвЕЩЯгавЛжжЗНЗЈЪЧгУCO2РДЩњВњШМСЯМзДМЁЃвЛЖЈЬѕМўЯТЗЂЩњЗДгІЃК
CO2(g)+3H2(g) ЃНCH3OH(g)+H2O(g) ЁїH1
ЃЈ1ЃЉвбжЊЃК 2CO(g) +O2(g) ЃН2CO2(g) ЁїH2
2H2(g)+O2(g) ЃН2H2O(g) ЁїH3
дђЁЁCO(g) + 2H2(g) CH3OH(g)ЁЁЕФЁЁЁїHЃН ЁЃ
ЃЈ2ЃЉгЩCOКЯГЩМзДМЪБЃЌCOдкВЛЭЌЮТЖШЯТЕФЦНКтзЊЛЏТЪгыбЙЧПЕФЙиЯЕШчЯТЭМЫљЪОЁЃ

ИУЗДгІІЄH 0ЃЈЬюЁА>ЁБЛђЁА <ЁБЃЉЁЃЪЕМЪЩњВњЬѕМўПижЦдк250ЁцЁЂ1.3ЁС104kPaзѓгвЃЌбЁдёДЫбЙЧПЕФРэгЩЪЧ ЁЃ
ЃЈ3ЃЉвЛЖЈЮТЖШЯТЃЌЯђ2LУмБеШнЦїжаМгШы1mol CH3OH (g)ЃЌ
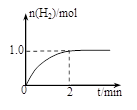
ЗЂЩњЗДгІЃКCH3OH(g) CO(g) + 2H2(g)ЃЌH2ЮяжЪЕФСПЫц
ЪБМфЕФБфЛЏШчгвЭМЫљЪОЁЃ
0~2 minФкЕФЦНОљЗДгІЫйТЪv(CH3OH)= ЁЃ
ИУЮТЖШЯТЃЌCO(g) + 2H2(g) CH3OH(g)ЕФЦНКтГЃЪ§
K= ЁЃ
ЯрЭЌЮТЖШЯТЃЌШєПЊЪММгШыCH3OH(g)ЕФЮяжЪЕФСПЪЧдРДЕФ2БЖЃЌдђ ЪЧдРДЕФ2БЖЁЃ
aЃЎЦНКтГЃЪ§ bЃЎCH3OHЕФЦНКтХЈЖШ cЃЎДяЕНЦНКтЕФЪБМф dЃЎЦНКтЪБЦјЬхЕФУмЖШ
ЃЈ4ЃЉвдCH3OHЮЊШМСЯЃЈвдKOHШмвКзїЕчНтжЪШмвКЃЉПЩжЦГЩCH3OHШМСЯЕчГиЁЃ
ЂйГфШыCH3OHЕФЕчМЋЮЊ МЋЃЛ
ЂкИКМЋЗДгІЕФЕчМЋЗДгІЪНЮЊ ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК2013НьМЊСжЪЁИпЖўЩЯбЇЦкжЪСПМьВтЛЏбЇЪдОэ ЬтаЭЃКЬюПеЬт
МзДМЪЧвЛжжгХжЪЕФвКЬхШМСЯЃЌCOКЭCO2ОљПЩгУгкКЯГЩМзДМЁЃ
ФПЧАЙЄвЕЩЯгавЛжжЗНЗЈЪЧгУCO2РДЩњВњШМСЯМзДМЁЃвЛЖЈЬѕМўЯТЗЂЩњЗДгІЃК
CO2(g) +3H2(g) ЃНCH3OH(g)+H2O(g) ЁїH1
ЃЈ1ЃЉвбжЊЃК 2CO(g) +O2(g) ЃН2CO2(g) ЁїH2
2H2(g)+O2(g) ЃН2H2O(g) ЁїH3
дђЁЁCO(g) + 2H2(g)  CH3OH(g)ЁЁЕФЁЁЁїHЃН
ЁЃ
CH3OH(g)ЁЁЕФЁЁЁїHЃН
ЁЃ
ЃЈ2ЃЉгЩCOКЯГЩМзДМЪБЃЌCOдкВЛЭЌЮТЖШЯТЕФЦНКтзЊЛЏТЪгыбЙЧПЕФЙиЯЕШчЯТЭМЫљЪОЁЃ

ИУЗДгІІЄH 0ЃЈЬюЁА>ЁБЛђЁА <ЁБЃЉЁЃЪЕМЪЩњВњЬѕМўПижЦдк250ЁцЁЂ1.3ЁС104kPaзѓгвЃЌбЁдёДЫбЙЧПЕФРэгЩЪЧ ЁЃ
ЃЈ3ЃЉвЛЖЈЮТЖШЯТЃЌЯђ2LУмБеШнЦїжаМгШы1mol CH3OH (g)ЃЌ

ЗЂЩњЗДгІЃКCH3OH(g)
 CO(g) + 2H2(g)ЃЌH2ЮяжЪЕФСПЫц
CO(g) + 2H2(g)ЃЌH2ЮяжЪЕФСПЫц
ЪБМфЕФБфЛЏШчгвЭМЫљЪОЁЃ
0~2 minФкЕФЦНОљЗДгІЫйТЪv(CH3OH)= ЁЃ
ИУЮТЖШЯТЃЌCO(g) + 2H2(g)  CH3OH(g)ЕФЦНКтГЃЪ§
CH3OH(g)ЕФЦНКтГЃЪ§
K= ЁЃ
ЯрЭЌЮТЖШЯТЃЌШєПЊЪММгШыCH3OH(g)ЕФЮяжЪЕФСПЪЧдРДЕФ2БЖЃЌдђ ЪЧдРДЕФ2БЖЁЃ
aЃЎЦНКтГЃЪ§ bЃЎCH3OHЕФЦНКтХЈЖШ cЃЎДяЕНЦНКтЕФЪБМф dЃЎЦНКтЪБЦјЬхЕФУмЖШ
ЃЈ4ЃЉвдCH3OHЮЊШМСЯЃЈвдKOHШмвКзїЕчНтжЪШмвКЃЉПЩжЦГЩCH3OHШМСЯЕчГиЁЃ
ЂйГфШыCH3OHЕФЕчМЋЮЊ МЋЃЛ
ЂкИКМЋЗДгІЕФЕчМЋЗДгІЪНЮЊ ЁЃ
ВщПДД№АИКЭНтЮі>>
ЙњМЪбЇаЃгХбЁ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com