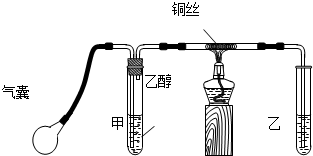
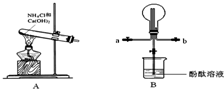
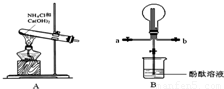
ijС��������ͼװ���Ʊ���������̽�����������ʣ�����������ȥ������ش�

��1��ʵ�����Ʊ������Ļ�ѧ����ʽΪ
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3+2H
2O
2NH
4Cl+Ca��OH��
2CaCl
2+2NH
3+2H
2O
��
��2����Bװ���ռ�����ʱ������ѡ�����Ľ�����
a
a
���a����b������
��3��Bװ������ƿ���ռ�����������ʹ֮�γ���Ȫ����IJ���������
���·�ֹˮ�У����֣�����ë����������ƿ��ʹ�����������ͣ��ϳ��������ڿ�����������ˮ�Ӵ��γ���Ȫ
���·�ֹˮ�У����֣�����ë����������ƿ��ʹ�����������ͣ��ϳ��������ڿ�����������ˮ�Ӵ��γ���Ȫ
�����۲쵽װ��B�е���ƿ�ڲ����˺�ɫ��Ȫ����˵���������е�������
��������ˮ����ˮ��Ӧ���ɼ�
��������ˮ����ˮ��Ӧ���ɼ�
��
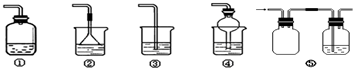
��4��Ϊ��ֹ������Ⱦ������װ�ã�ʢ�ŵ�Һ���Ϊˮ�����������ն��ఱ������
�ڢܢ�
�ڢܢ�
������ţ���

��5�������ڴ��������²��Ҽ���ʱ�ᱻ�������������ǹ�ҵ������ĵ�һ����Ӧ��д���÷�Ӧ�Ļ�ѧ����ʽ
��
��6�������������ڳ����¿ɿ��ٷ�Ӧ���ɵ�����2NH
3+3Cl
2=N
2+6HCl���÷�Ӧ�����ڼ��黯�������������Ƿ�й©����Ϊ����������й©���ð�������ʱ�����������˹����з�����Ӧ��Cl
2��NH
3������ȷ�ΧΪ
��1.5
��1.5
��





 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�