
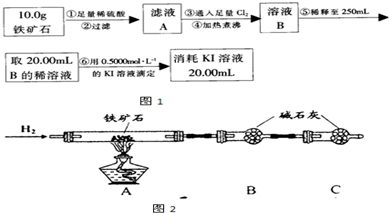
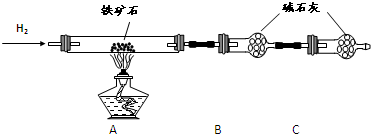
����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����

A B C
������ʯ�к������IJⶨ
�� ����ͼ��װ���������װ�õ������ԣ�
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�� ����˵����ܿڴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ���
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ ��
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ ��
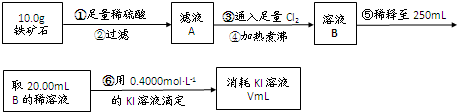
������ʯ�к������IJⶨ
��1����������������� ��
��2����������õ��IJ����������ձ�������������ͷ�ιܡ� ��
��3�������йز���IJ�����˵����ȷ���� ��

a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���Һ��ϴ
e���ζ������У��۾�ע�ӵζ�����Һ��仯
f���ζ�������30s����Һ���ָ�ԭ������ɫ�ٶ���
��4�����ζ�����������0.5000mol��L−1KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ ��
���ɢ���������������ʯ������������Ļ�ѧʽΪ ��
��14�֣�
��1����ֹ�����е�ˮ������CO2����B�У�Ӱ��ⶨ�������2�֣�
��2��24% ��2�֣�
��1��������Һ���ܽ�Ĺ�����Cl2 ��2�֣�
��2��250mL����ƿ ��2�֣���δ��250mL�������֣�
��3��df ��2�֣� ��4��70% ��2�֣���. Fe5O6 ��2�֣�
��������
���������
��1��B�еļ�ʯ���������û���Ӧ���ɵ�ˮ�ģ�Ϊ�˷�ֹ�����ɷֶ�ʵ���Ӱ�죬Ҫ��һ��װ�����տ����е�ˮ�Լ�������̼��
��2����Ԫ�صİٷֺ���Ϊ��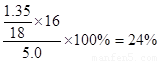
��1��ͨ�������Cl2��Ϊ�˽���Ԫ��ȫ����ΪFe3+������Ϊ�˷�ֹ������������KI��Ӧ��ʹ�ⶨ���ƫ�ߣ�����Ҫ����Һ��и�����Һ���ܽ�Ĺ�����Cl2��
��2�����������Һ�����ƣ�ϡ�ͣ�����֪�ô�Ӧ����250ml������ƿ��
��3��a����ˮ��Fe3+��Ϊ��ɫ���ζ���������ɫ�����Ա仯����a����
b��2Fe3+ + 2I− = 2Fe2+ + I2���ζ�һ��ʼ������I2��ʹ������Һ��������ָʾ�յ㡣��ȷ��ָʾ��Ӧ��ΪKSCN����ɫ�仯ΪѪ��ɫ��Ϊ��ɫ����b����
c���ζ���������ˮϴ�Ӻ�����ô�װҺ��ϴ�����װҺ����c����
e���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ����e����
f���ζ������У��۾�ע����ƿ����Һ��ɫ�仯����f����
��4��������ȡ��Һ����Fe3+�������������KI��Һ�������ȣ���Ϸ���ʽ��֪��c(Fe3+)=c(KI)=0.5mol��L−1��������Ԫ�صİٷֺ���Ϊ��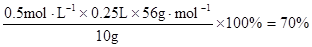
����Ϊ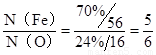 ����������ʯ������������Ļ�ѧʽΪFe5O6
����������ʯ������������Ļ�ѧʽΪFe5O6
���㶨λ��������һ��̽���������������������������ʵĺ���֪ʶ��һ���ۺϿ����⣬����ѧ�������ͽ��������������ۺ���ǿ���Ѷȴ�


 ����������������ϵ�д�
����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ������У����5���������������ۺϻ�ѧ ���ͣ������
��18�֣�����ʯ�ǹ�ҵ��������Ҫԭ��֮һ������Ҫ�ɷ�Ϊ����������������в�����Ԫ�غ���Ԫ�أ������ʲ���H2SO4��Ӧ����ij�о���ѧϰС���ij����ʯ������������Ļ�ѧʽ����̽����
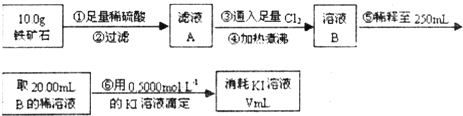
������ʯ�к������IJⶨ
�� ����ͼ��װ���������װ�õ������ԣ�
�� ��5.0g����ʯ����Ӳ�ʲ������У�װ��B��C�е�ҩƷ��ͼ��ʾ���г�������ʡ�ԣ���
�۴���˵����ܿڴ����ϵػ���ͨ��H2����Cװ�ó��ڴ�H2�鴿��ȼA���ƾ��ƣ�
�� ��ַ�Ӧ�����ƾ��ƣ��ٳ���ͨ����������ȫ��ȴ��
��1��װ��C������Ϊ ��
��2����ķ�Ӧ��װ��B����1.35g��������ʯ�����İٷֺ���Ϊ ��
��3������H2����CO�����貹�� װ�á�
������ʯ�к������IJⶨ
��1����ҺA����Ԫ�ؿ��ܵĴ�����ʽ ����������Լ���ѡ������Լ������ʵ����֤A����Ԫ�صĴ�����ʽ���Լ�Ϊ�� ��
a������KMnO4��Һ b��NaOH��Һ c��KSCN��Һ d����ˮ
��2����������������� ��
��3����������õ��IJ��������У� ��
��4�������йز���IJ�����˵����ȷ���� ��
a����Ϊ��ˮΪ��ɫ�����Եζ������в����ָʾ��
b���ζ������п����õ�����Һ��Ϊָʾ��
c���ζ���������ˮϴ�Ӻ����ֱ��װҺ
d����ƿ����Ҫ�ô���ҹ��ϴ
e���ζ�ʱ�����ֿ��Ƶζ��ܣ�����ҡ����ƿ
f���ζ������У��۾�ע�ӵζ�����Һ��仯
g���ζ�������Һ���ȶ����ٶ���
��5�����ζ�����������0.5000mol��L?1��KI��Һ20.00mL��������ʯ�����İٷֺ���Ϊ ��
���ɢ���������������ʯ������������Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com