
�㱻��ɱ�������ڵ�ATP�����ɾ�����ζ�ļ����ᣬ����������ø��ACP�����������ֽ��������ζ�½���Ϊ���о�����ı��ʷ������о��ߴӲ��㡢�t��������з���õ�ACP�����Ը�ø���Խ�����ϵ���о������ʵ�������¡������й�������ȷ����
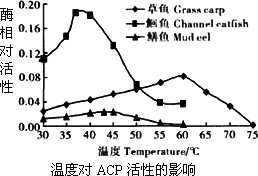

��ͬ�����ACP���Զ��������¶ȵ�����������
����ɱ����t��ŵ�37�����ҵĻ�����һ��ʱ���ܱ�������ζ
����ɱ��IJ���ŵ�����Ũ�ȵ�Ca2+��Һ����ζ�½����ٶȻ����
Zn+��ʹ�����������ζ�½��ٶȶ�����
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
�㱻��ɱ�������ڵ�ATP�����ɾ�����ζ�ļ����ᣬ����������ø(ACP)���������ֽ��������ζ�½���Ϊ���о�����ı��ʷ������о��ߴӲ��㡢�t��������з���õ�ACP�����Ը�ø���Խ�����ϵ���о������ʵ�������¡������й�������ȷ����
 ACP�ڲ�ͬŨ�Ƚ��������е����ø����
ACP�ڲ�ͬŨ�Ƚ��������е����ø����
| ���� ���� | Ũ�� ��mmol/L�� | ��Ի��ԣ����� | ||
| ���� | �t�� | ���� | ||
| Na�� | 30 | 100.83 | 101.47 | 96.03 |
| Zn+ | 1 | 112.38 | 116.06 | 158.13 |
| Ca2+ | 5 | 65.21 | 96.18 | 88.18 |
A����ͬ�����ACP���Զ��������¶ȵ�����������
B������ɱ����t��ŵ�37�����ҵĻ�����һ��ʱ���ܱ�������ζ
C������ɱ��IJ���ŵ�����Ũ�ȵ�Ca2+��Һ����ζ�½����ٶȻ����
D�� Zn+��ʹ�����������ζ�½��ٶȶ�����
��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ���㽭ʡ�����и����ڶ����ʼ죨���ۣ����ﲿ�� ���ͣ�ѡ����
�㱻��ɱ�������ڵ�ATP�����ɾ�����ζ�ļ����ᣬ����������ø(ACP)���������ֽ��������ζ�½���Ϊ���о�����ı��ʷ������о��ߴӲ��㡢�t��������з���õ�ACP�����Ը�ø���Խ�����ϵ���о������ʵ�������¡������й�������ȷ���� 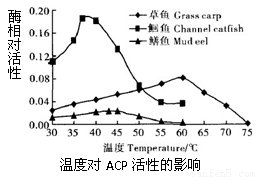
ACP�ڲ�ͬŨ�Ƚ��������е����ø����
|
���� ���� |
Ũ�� ��mmol/L�� |
��Ի��ԣ����� |
||
|
���� |
�t�� |
���� |
||
|
Na�� |
30 |
100.83 |
101.47 |
96.03 |
|
Zn+ |
1 |
112.38 |
116.06 |
158.13 |
|
Ca2+ |
5 |
65.21 |
96.18 |
88.18 |
A����ͬ�����ACP���Զ��������¶ȵ�����������
B������ɱ����t��ŵ�37�����ҵĻ�����һ��ʱ���ܱ�������ζ
C������ɱ��IJ���ŵ�����Ũ�ȵ�Ca2+��Һ����ζ�½����ٶȻ����
D�� Zn+��ʹ�����������ζ�½��ٶȶ�����
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ���ѡ��
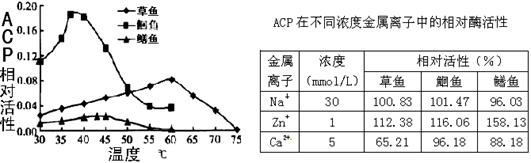
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�㱻��ɱ�������ڵ�ATP�����ɾ�����ζ�ļ����ᣬ����������ø(ACP)���������ֽ��������ζ�½���Ϊ���о�����ı��ʷ������о��ߴӲ��㡢�t��������з���õ�ACP�����Ը�ø���Խ�����ϵ���о������ʵ�������¡������й�������ȷ���� 
ACP�ڲ�ͬŨ�Ƚ��������е����ø����
| ���� ���� | Ũ�� ��mmol/L�� | ��Ի��ԣ����� | ||
| ���� | �t�� | ���� | ||
| Na�� | 30 | 100.83 | 101.47 | 96.03 |
| Zn+ | 1 | 112.38 | 116.06 | 158.13 |
| Ca2+ | 5 | 65.21 | 96.18 | 88.18 |
A����ͬ�����ACP���Զ��������¶ȵ�����������
B������ɱ����t��ŵ�37�����ҵĻ�����һ��ʱ���ܱ�������ζ
C������ɱ��IJ���ŵ�����Ũ�ȵ�Ca2+��Һ����ζ�½����ٶȻ����
D�� Zn+��ʹ�����������ζ�½��ٶȶ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com