
�о��ֽ���ά�ص�������������ڹ�ҵ�����е�Ӧ�����Ż��������塣��ش�
��1�������Ƿ�����ά�طֽ����ʵ�����̣��벹ȫ��ȱ���ݣ�
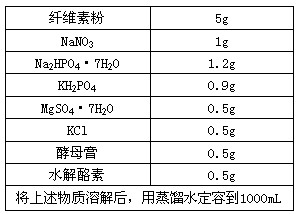
����ȡ������ ���ݶ�ϡ�͡��� ���� ��
| ��ά�ط� | 5 g |
| NaN03 | 1 g |
| Na2HP04��7H 20 | 1.2 g |
| KH2P04 | 0.9 g |
| MgS04��7H20 | 0.5 g |
| KCl | 0.5 g |
| ��ĸ�� | 0.5 g |
| ˮ������ | 0.5 g |
| �����������ܽ��������ˮ���ݵ�1000 mL | |
��1��ѡ������ ���ֵ�������ά�طֽ������������ ��ѡ������Ȧ�ľ��䣨ÿ��1�֣�
��2��Һ����������1�֣��ֽܷ���ά�ص�������ܴ�����ֳ���������������棨2�֣� ������ά�طֽ����Ũ�ȣ�ȷ���ܷ���õ�����ľ��֣�2�֣�
��3���չ��� ��1�֣�
��4��ѡȡδ���ֵ��������������Ʒ��������ͬʱ���������������������δ������������û�о���������˵��������û�б��Ӿ���Ⱦ����2�֣�
��5����ά��ø �����ǣ�ÿ��1�֣�
��6������������ϸ������һ��ʱ��ƽ���Ϲ۲쵽��ֻ��һ�����䣨2�֣�
��������������ŷ�����ά�طֽ����ʵ�������ǣ�����ȡ����ѡ������ ���ݶ�ϡ�͡����ֵ�������ά�طֽ�����������ϡ�����ѡ������Ȧ�ľ��䡣
�ƽ����������ܽ���õ�������ˮ���ݣ��ɼ���Һ��������������������ά��ΪΨһ̼Դ��ֻ���ֽܷ���ά�ص�ϸ�����ܴ��Ӷ��ﵽɸѡ��Ŀ�ġ��������С����������ά�ص����������Ը��õشﵽɸѡ��Ŀ�ġ�
�Ǹչ�����һ��Ⱦ�ϣ�������������ά�������Ķ��������γɺ�ɫ����������ܺ�ˮ������ά���Ǻ������Ƿ������ַ�Ӧ��
�Ƚ�δ���ֵ����������������������������û�о���������֤��û���Ӿ���Ⱦ��������
�ɵ��������е���ά�ر�ϸ�������ڵ���ά��ø�ֽ�չ��졪��ά�ظ���������γɣ������������л��������ά�ؽ����Ϊ���ĵ���Ȧ��
������ϡ�͡�Ϳ���ȹ��̲�ǡ�������ܻ�ʹ���������ص���һ������ʹ����ƫ�͡�
�������������ķ����������Ҫ��ΪA����
�����������Ѷ�һ�㣬Ҫ��ѧ������һ���Ļ���֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
�о��ֽ���ά�ص�������������ڹ�ҵ�����е�Ӧ�����Ż��������塣��ش�
��1�������Ƿ�����ά�طֽ����ʵ�����̣��벹ȫ��ȱ���ݣ�
����ȡ������ ���ݶ�ϡ�͡��� ���� ��
| ��ά�ط� | 5 g |
| NaN03 | 1 g |
| Na2HP04��7H 20 | 1.2 g |
| KH2P04 | 0.9 g |
| MgS04��7H20 | 0.5 g |
| KCl | 0.5 g |
| ��ĸ�� | 0.5 g |
| ˮ������ | 0.5 g |
| �����������ܽ��������ˮ���ݵ�1000 mL | |
��2���ұߵ��������䷽�Ǣٹ�����ʹ�õ������������䷽��Һ�����������ǹ����������� �����������Ƕ���ά�طֽ����������ѡ��
��
���̢ٵ�Ŀ����
��
��3�����̢��õ����������м�����һ��Ⱦ���� ��
��4�����֤�����̢��õ���������û�б��Ӿ���Ⱦ��
��
��5��������Χ������Ȧ˵����ά�طֽ���ܷ��� �����հ���ά�طֽ�� ��
��6������ƽ��������Է������ά�طֽ����Ŀͳ��ʱ��ͳ�Ƶľ����������Ȼ����ʵ����Ŀ�ͣ�������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2010-2011ѧ��ɽ��ʡ�����ڶ�����У���������ۣ����ﲿ�� ���ͣ��ۺ���
�о��ֽ���ά�ص�������������ڹ�ҵ�����е�Ӧ�����Ż��������塣��ش�
��1�������Ƿ�����ά�طֽ����ʵ�����̣��벹ȫ��ȱ���ݣ�
����ȡ������ ���ݶ�ϡ�͡��� ���� ��
|
��ά�ط� |
5 g |
|
NaN03 |
1 g |
|
Na2HP04��7H 20 |
1.2 g |
|
KH2P04 |
0.9 g |
|
MgS04��7H20 |
0.5 g |
|
KCl |
0.5 g |
|
��ĸ�� |
0.5 g |
|
ˮ������ |
0.5 g |
|
�����������ܽ��������ˮ���ݵ�1000 mL |
��2���ұߵ��������䷽�Ǣٹ�����ʹ�õ������������䷽��Һ�����������ǹ����������� �����������Ƕ���ά�طֽ����������ѡ��
��
���̢ٵ�Ŀ����
��
��3�����̢��õ����������м�����һ��Ⱦ���� ��
��4�����֤�����̢��õ���������û�б��Ӿ���Ⱦ��
��
��5��������Χ������Ȧ˵����ά�طֽ���ܷ��� �����հ���ά�طֽ�� ��
��6������ƽ��������Է������ά�طֽ����Ŀͳ��ʱ��ͳ�Ƶľ����������Ȼ����ʵ����Ŀ�ͣ�������Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��ģ���� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
�о��ֽ���ά�ص�������������ڹ�ҵ�����е�Ӧ�����Ż��������塣��ش�
��1��������ά�طֽ����ʵ�����̣�����ȡ�����ݶ�ϡ�͡�����Ʒ���ֵ�������ά�طֽ�����������ϡ���ѡ������Ȧ�ľ��䡣����ʵ���е�����������ѡ����������������һ���������飬���Խ����е���ά�طۻ��� ����ά�طֽ����ɸѡ������ ������
��2�����֤��ѡ��������û�б��Ӿ���Ⱦ��
��3������ƽ��������Է������ά�طֽ����Ŀͳ��ʱ��ͳ�Ƶľ����������Ȼ����ʵ����Ŀ�ͣ�������Ϊ ��
��4�����̽���¶ȶ���ά��ø���Ե�Ӱ�죬�Ա����� ��Ӧ�ñ��� �Ȳ��䡣�����ٴ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com