

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�043
������ͼ�ش����⣺

��1��ͼ����ʾ�IJ����У���ˮ����͵���________��__________��ԭ����___________��
��2��ͼ�е�__________��ˮ����ߣ���ͼ����֪��һ����������ˮ�ֵĺ���Լռ���ص�____________��
��3�������ڵ�ˮ��Լռ�������ص�________��ͳ��Ϊ________����һ���ִ���������ϸ����Һ���⣬����Ĺ��������ϸ����Һ����____��_______��___________��
��4������ѪҺ��pHֵ�仯��Χ��С��ֻ����7.0��7.8�ķ�Χ�ڱ仯������������Σ�ա�ʵ��֤������50ml��ѪҺ�м���1�Σ�0.05ml��10mol�� ������ʱ��pHֵ��7.4����7.2�������50ml��pHֵΪ7.4��NaOH��Һ�м���1�Σ�0.05ml��10mo1��
������ʱ��pHֵ��7.4����7.2�������50ml��pHֵΪ7.4��NaOH��Һ�м���1�Σ�0.05ml��10mo1��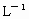 ������ʱ��pHֵ��Լ����Ϊ_________________��Ȼ������ѪҺ����___________�����á�
������ʱ��pHֵ��Լ����Ϊ_________________��Ȼ������ѪҺ����___________�����á�
��5��ע���õ�������ˮŨ��Ϊ_____________��ԭ����____________��
��6�������ײ������ں�����������ʹ�ڻ����е�pHֵ��_____________
��7������ˮ���������ڵ����ã�_________________________________
��8���ִ���ѧ�Ѷ��֤�����ö���տ��ķ�ˮ���������к��ģ���˵�������ɣ�_____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ����������� ���ͣ�071
������ͼ�ش����⣺

��1��ͼ����ʾ�IJ����У���ˮ����͵���________��__________��ԭ����___________��
��2��ͼ�е�__________��ˮ����ߣ���ͼ����֪��һ����������ˮ�ֵĺ���Լռ���ص�____________��
��3�������ڵ�ˮ��Լռ�������ص�________��ͳ��Ϊ________����һ���ִ���������ϸ����Һ���⣬����Ĺ��������ϸ����Һ����____��_______��___________��
��4������ѪҺ��pHֵ�仯��Χ��С��ֻ����7.0��7.8�ķ�Χ�ڱ仯������������Σ�ա�ʵ��֤������50ml��ѪҺ�м���1�Σ�0.05ml��10mol�� ������ʱ��pHֵ��7.4����7.2�������50ml��pHֵΪ7.4��NaOH��Һ�м���1�Σ�0.05ml��10mo1��
������ʱ��pHֵ��7.4����7.2�������50ml��pHֵΪ7.4��NaOH��Һ�м���1�Σ�0.05ml��10mo1�� ������ʱ��pHֵ��Լ����Ϊ_________________��Ȼ������ѪҺ����___________�����á�
������ʱ��pHֵ��Լ����Ϊ_________________��Ȼ������ѪҺ����___________�����á�
��5��ע���õ�������ˮŨ��Ϊ_____________��ԭ����____________��
��6�������ײ������ں�����������ʹ�ڻ����е�pHֵ��_____________
��7������ˮ���������ڵ����ã�_________________________________
��8���ִ���ѧ�Ѷ��֤�����ö���տ��ķ�ˮ���������к��ģ���˵�������ɣ�_____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012�����ʡ���һ�и�����һ���¿������Ծ� ���ͣ���ѡ��
��10�֣�ÿ��1�֣���ͼΪֲ���ij��Ҷ��ϸ���е�����Ĥ�ṹ���Լ�������������Ӧ�����ͼ�����ش�
��1��ͼ�ס����е���������Ĥ�ֱ������ �� ����ϸ��������,�ס�����������Ĥ���������������⣬�����ɲ��� ��
��2��ͼ����ø�Ļ�����λ�� ���������У��û�����λ������Լ�� �֡�
��3��Ӱ��ס�������Ĥ��������Ӧ����Ҫ�������طֱ��� �� ��
��4��ͼ���е�[H]ʵ������ ,ͼ���е�[H]��Ҫ������ �ķֽ⡣
��5�������ͼ����ø�ĵ����ƽ����Է�������Ϊn��ͨ����Ӧ�����γ�m���������������۵�������Է�������Ϊ�ֵ�C����C�������ļ�����Ŀ�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ��㶫ʡ�����и�һ��ѧ����ĩ�����Ծ� ���ͣ��ۺ���
��ͼ��A��B��ʾ����ϸ����������Ҫ���л����ͼ�ش��������⡣
��1��A��ϸ���ں��� ���л��ϸ���е��ܺ��������� ��
��2����ͼ����a����A�������Ƕ��a����ͨ�� �ķ�ʽ�����γɶ����������Ӷ��a���Ӽ�Ļ�ѧ�����ļ������ɱ�ʾΪ ��
��3�������������A��aԼ�� �֣�����ϸ����ÿ��a����Ŀ�ɰ���ǧ��a�����γɶ�����ʱ����ͬ����a�� ǧ�������������������۵���ʽ�����γ�A�� ǧ��������A���ӵĽṹ����������Ӷ���ϸ���ге����ֹ��ܡ�
��4����������Һ����A���ʣ������ײ����С���ⲡ����Һ�Ƿ���A���ʣ��ɰ���ҽ��ȷ�����ס����ʱ��Ӧѡ�õ��Լ��� ������A���ʷ������ã����� ɫ��Ӧ��
��5�������ڵ�B�����࣬�ֱ��� ������ ���Ⱦɫ����ϸ��Ⱦɫ��������ʾ������ϸ���еķֲ���B�Ļ�����ɵ�λb�������� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
����ͼʾ�ش����⣺

(1)ͼ����ʾ�IJ����У���ˮ����͵���__________��________��ԭ����________________________________________________________________________
________________________________________________________________________��
(2)ͼ�е�________��ˮ����ߣ���ͼ����֪��һ����������ˮ�ֵĺ���Լռ���ص�________��
(3)�����ڵ�ˮԼռ�������ص�________��ͳ��Ϊ________����һ���ִ���������ϸ����Һ���⣬����Ĺ��������ϸ����Һ����________��________��________��
(4)����ѪҺ��pH�仯��Χ��С��ֻ����7.35��7.45�ķ�Χ�ڱ仯������������Σ�ա�ʵ��֤������50 mL��ѪҺ�м���1��(0.05 mL)10 mol��L��1������ʱ��pH�������䡣��Ȼ������ѪҺ����____________�����á�
(5)ע���õ�������ˮŨ��Ϊ________��ԭ����__________________________________
________________________________________________________________________��
(6)�����ײ������ں�����������ʹ�ڻ����е�pH��________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com