
 ��
�� ���� ͼ�Т�Ƭ��ΪͬԴ���֣�ͬԴ������X��Y����Ⱦɫ���ϵĻ�������ͬ�ģ���1����2Ƭ��Ϊ��ͬԴ���֣���ͬԴ������״���Ŵ����Ա��������
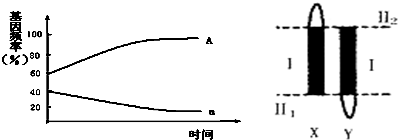
9��7���ϵ¶�������϶��ɵ��������ȣ����������ij�������������Ի���ͬ����ʱ���ܺϳɣ���˫�ײ��ܺϳɸ����ʣ����ң�G��g��λ�ڢ�Ƭ���ϣ���˫�Ļ�����ΪEEXgYg��eeXGXG��EEXgXg��eeXGYG��F2�����Ի�������Ϊ9����������Ϊ6��˫���Ի���Ϊ1������Ӷ������ܺϳɸ������벻�ܺϳɸ����ʵı���Ϊ9��7��
��� �⣺��1����������Ⱥ�д��۸�����п����Ͳ������ĸ�����ڣ����Ƹ�ֲ��Ŀ���������λ��ͼ�еĢ�1�Σ�
��2���ӽ��ױ��ı�����Ϊ���Բ����������Կ�������Ϊ�����ӣ������Ƹ���״�Ļ���λ��ͼ�еĢ�2Ƭ�ϣ��ױ�ΪXdXd��XDY�����Ӵ�ΪXDXd�����Կ�������XdY�����Բ���������
��3�����ڲ������������Ⱦɫ�����ΪXXY�������������ΪXdXdY�����ױ��ǿ�������XDY�����ԣ������ԭ���Dz����������ڼ��������γ����ӵĹ����У��������ͬԴȾɫ��δ����������ڽ���Ⱦɫ��δ���룮
��4������������ƶ����ĺϳɣ����ֿ�����״���ں�����ϳɶ�����������Ҫ��������ģ��mRNA��ת�˹���tRNA��ԭ�ϰ����ᡢ����ø������ATP��
��5����Ƭ����X��Y��ͬԴ������Ϊһ�Գ�Ⱦɫ�壬���ױ�ΪEEgg��eeGG����һ��ΪEeGg���Ӷ�����EGΪ$\frac{9}{16}$��ʣ��Ϊ$\frac{7}{16}$�����������ױ��Ļ����Ϳ���ΪEEXgYg��eeXGXG��EEXgXg��eeXGYG��
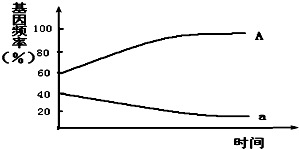
��6����������������������ΪAA=Aa��aa����A������a�����٣�A���Ϊ24%+$\frac{1}{2}$��72%=60%��a���Ϊ4%+$\frac{1}{2}$��72%=40%������ͼΪ��
�ʴ�Ϊ��
��1����1
��2�����Բ����������Կ��� ����Ϊ����������Ϊ������
��3���������ͬԴȾɫ��δ����������ڽ���Ⱦɫ��δ����
��4��mRNA��tRNA�������ᡢø��ATP
��5��EEXgYg��eeXGXG��EEXgXg��eeXGYG
��6��������ͼ��A���60%��a���40%������������Ҫ��ȷ����
���� ���⿼���˻���������϶��ɵ�Ӧ�úͰ����Ŵ������֪ʶ�����ڿ��鿼����������ѧ֪ʶ��۵㣬ͨ���Ƚϡ��������ۺϵȷ�����ijЩ����ѧ������н��͡������������������жϻ�ó���ȷ�Ľ��۵�������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2015-2016ѧ�꽭��ʡ�����и߶�����ĩ�����Ծ��������棩 ���ͣ��ۺ���
��2015��•������ĩ��ȡ������ʢ����Ҷ����ֱ��Ϊ1cm��������СԲƬ���ɣ�ע��ܿ���Ҷ���������СҶԲƬ�ڵ����壬���ںڰ�������ˮ����ʹСҶԲƬϸ����϶����ˮ��Ȼ��ƽ����װ��ʢ�е����ĺ���ͬŨ�ȵ�CO2��Һ��С�ձ��У����ڹ��º㶨�����˵������£���ø���СҶԲƬ�ϸ���Һ������ƽ��ʱ�䣬����¼������Ƴ�������ͼ1��

��1����aŨ����ȣ�bŨ��ʱСҶԲƬҶ��ϸ���������屡Ĥ��[H]���������� ����족������ȡ�������������ʱСԲƬ��Ҷ��ϸ�����ܲ���ATP��ϸ������ �� ��
��2��ͼ1�У���CO2Ũ�ȴ���cʱ������CO2Ũ�ȵ�����СҶԲƬ���л���Ļ������� ����ӿ족�������䡱����������
��3����ȡ������ͬ��СҶԲƬ�ֱ�������ҺCO2Ũ��Ϊa��С�ձ��У�ѡ��40W��̨����Ϊ��Դ��ͨ���ı��Դ��С�ձ�֮��ľ������ʵ�飨ʵ���¶ȱ�����ͬ�����ˣ�������ʵ�������Ƴ�ͼ2���ߣ�X1��0����
�ٸ�ʵ���Ŀ���� ��
������B�㾻������ʵ���Ҫ��������� ��C���ʾ ʱ��Դ��С�ձ�֮��ľ��룮
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ���߶����ڹ���ϸ������������ | |
| B�� | �������ߵĻ����Ǽܶ���̼�� | |
| C�� | �����ڵ����ʵ�����ϳ��о�����Ҫ���� | |
| D�� | ���ߵ����Ԫ���ж�����C��H��O��N��P |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| ��Ŀ | H2O | NH4+ | K+ | Ca2+ | PO43- |
| ������ | 0% | 83% | 72% | 97% | 84% |
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢۢ� | D�� | ����ȷ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ͻ�����ֱ�ӿ��Ƹü���������Ҫת¼�ͷ������ | |
| B�� | ��ͨ������ø�ĺϳɣ����ƴ�л���̴Ӷ������������״ | |
| C�� | ͻ������ڿ�����״ʱ���е�����ת¼���� | |
| D�� | ������������빲��ʧ��ëϸѪ������֢��������ԭ����ͬ |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ������
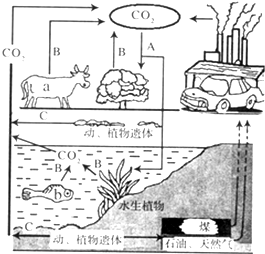 ������������ȫ�������ЧӦ����Щ����̨�籩�겻�ϣ���Щ��������Ӹɺ�����2009��籾�������Ϲ�����仯�����ϣ��й�������ŵ����2020�굥λGDP��̼�ŷ�������2005���½�40%--45%������̼���ѳ�Ϊÿһ���˵����Σ�������̼����ָ��������������ֱ�ӻ��ӽ������������ģ��Ӷ�����̼���ŷţ���ͼ��ij����ѭ������ʾ��ͼ�������������ݣ��ش����⣮
������������ȫ�������ЧӦ����Щ����̨�籩�겻�ϣ���Щ��������Ӹɺ�����2009��籾�������Ϲ�����仯�����ϣ��й�������ŵ����2020�굥λGDP��̼�ŷ�������2005���½�40%--45%������̼���ѳ�Ϊÿһ���˵����Σ�������̼����ָ��������������ֱ�ӻ��ӽ������������ģ��Ӷ�����̼���ŷţ���ͼ��ij����ѭ������ʾ��ͼ�������������ݣ��ش����⣮�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ������
 ij�صij�����Ҷ����ɭ�ִ�����ƻ�������һ��ʱ��õس�����Ҷ�����Իָ�����ͼΪ�ָ�������Ⱥ������IJ�ͬ�μ���ֲ����ɣ�
ij�صij�����Ҷ����ɭ�ִ�����ƻ�������һ��ʱ��õس�����Ҷ�����Իָ�����ͼΪ�ָ�������Ⱥ������IJ�ͬ�μ���ֲ����ɣ��鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | 2��46 | B�� | 4��46 | C�� | 8��92 | D�� | 1��92 |
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�ѡ����
| A�� | ��1+1��2����������̬���̵�������ԭ�� | |
| B�� | ���������ˮ����Υ�������ֶ�����ԭ�� | |
| C�� | �ƹ㡰�����Ѻá������͵���Ⱦ����������գ����Լ�����Ⱦ�IJ������������л��� | |
| D�� | Ϊ�ٽ�������̬�����Ļָ����ɲ����˹������������ֲũ����Ĵ�ʩ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com