
��5�֣�ϸ���ڴ�л�����У�������������⣬����ϸ���ж������ã��������й�������ø�Ĵ��ڿ��Խ���ֽ�Ϊ�������ʡ����������һ��ʵ�飬���ش��й����⣺
| �Թܱ�� | ������ | ���� | ���� |
| A | 2mL H2O2+2��FeCl3 | 30�� | �ų��������� |
| B | 2mL H2O2+2�θ�����ĥҺ | 30�� | �ų��������� |
| C | 2mL H2O2+2������ĸ�����ĥҺ | ��к���ȴ��30�� | ������ |
| D | 2mL H2O2+2���䶳�ĸ�����ĥҺ | 0�� | ������ |
| E | 2mL H2O2+2���˵�������Һ | 30�� | ������ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ�� ���ͣ�
ϸ���ڴ�л�����У�������������⣬����ϸ���ж������ã��������й�������ø�Ĵ��ڿ��Խ���ֽ�Ϊˮ��������������Կ������·����ⶨ���ô�����������Ƴ�СԲƬ�������ں�ø�Ĺ��������Һ�У�ʵ�鿪ʼʱ��СԲƬ��������Һ�ĵײ����������ḡ��Һ�棬��¼СԲƬ�ϸ���ʱ�䣬��ʱ��ĵ�������ʾ��������Ĵ����ԡ�Ϊ�о���Ӧ��Ũ�ȶ�ø�����Ե�Ӱ�죬ʵ���ڲ�ͬŨ�ȵĹ���������Һ���ظ����У�ʵ������ͼ��ʾ���������ش��������⣺
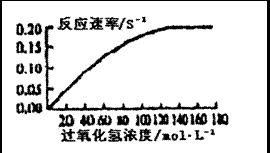
��1��СԲ��ֽƬ���е�һ��ʱ���ʼ�ϸ���ԭ���� ��
��2��ʵ���й���������Һ�û���Һ��Ŀ���� ��
��3������ͼ�е�����������Ӧ��Ũ�����������ø�����ʼ�Ĺ�ϵ
��
��4��������Ũ�ȳ���140mol��L��1ʱ����Ӧ���ʲ��ټӿ��ԭ���� ��
��5��ijͬѧ���һ��̽����ͬ�¶ȶԹ�������ø���Ե�Ӱ�죬�������ʵ�飬����
��ȡ6֧�Թֱܷ���ΪA1��A2��B1��B??2��C1��C2����A1��B1��C1�м�������Ĺ������⡢����Һ��СԲ��ֽƬ����A2��B2��C2�м������������Ĺ�������ø��Һ����������²��裺
��
��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�
ϸ���ڴ�л�����У�������������⣬����ϸ���ж������ã��������й�������ø�Ĵ��ڿ��Խ���ֽ�Ϊˮ��������������Կ������·����ⶨ���ô�����������Ƴ�СԲƬ�������ں�ø�Ĺ��������Һ�У�ʵ�鿪ʼʱ��СԲƬ��������Һ�ĵײ����������ḡ��Һ�棬��¼СԲƬ�ϸ���ʱ�䣬��ʱ��ĵ�������ʾ��������Ĵ����ԡ�Ϊ�о���Ӧ��Ũ�ȶ�ø�����Ե�Ӱ�죬ʵ���ڲ�ͬŨ�ȵĹ���������Һ���ظ����У�ʵ������ͼ��ʾ���������ش��������⣺
|
��1��СԲ��ֽƬ���е�һ��ʱ���ʼ�ϸ���ԭ���� ��
��2��ʵ���й���������Һ�û���Һ��Ŀ���� ��
��3������ͼ�е�����������Ӧ��Ũ�����������ø�����ʼ�Ĺ�ϵ
��
��4��������Ũ�ȳ���140mol?L��1ʱ����Ӧ���ʲ��ټӿ��ԭ���� ��
��5��ijͬѧ���һ��̽����ͬ�¶ȶԹ�������ø���Ե�Ӱ�죬�������ʵ�飬����4�֣���
��ȡ6֧�Թֱܷ���ΪA1��A2��B1��B2��C1��C2����A1��B1��C1�м�������Ĺ������⡢����Һ��СԲ��ֽƬ����A2��B2��C2�м������������Ĺ�������ø��Һ����������²��裺
��
��
�� ��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2012-2013�긣����Դ��һ��ѧ��һ�ڶ����¿������Ծ����������� ���ͣ��ۺ���
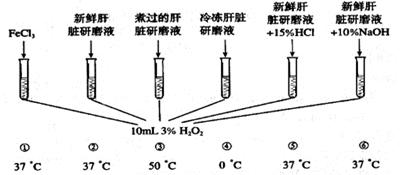
H2O2������Ư�ף�Ҳ������ϴС���˿ڣ�ϸ���ڴ�л������Ҳ�����H2O2������ϸ���ж������ã��������й�������ø����ʹ��ֽ�Ϊ�����ʡ�
�������ͼ�е�ʵ�飬���ش��й����⣺��14�֣�
��1���ڢ١����Թ��У����Ȳ������ݵ����Թ� ����λʱ���ڲ��������������Թ� ��
��2�����Թܢ��У�ѡ�����ʸ�����ʵ�飬����Ϊ���ʸ����У���������
A.����������ࡡB.��������ø�ӷ� C.��ø������ࡡD.��������ø���һ���ǿ
(3)��ʵ����ȡ���ʸ���,Ѹ�ټ���Ͷ���Թܢ���,�����Ŀ���ǣ�������
A.��ֹ��������ø�ӷ�����������B.��ֹ��������ø�ֽ�
C.��ֹϸ���й�������ø���ͷš�D.ʹ��������ø��H2O2��ֽӴ�
��4���ȽϢٺ͢ں��Թܣ�˵��ø�� ��
��5���ȽϢںۢ͢ܢݢ��Թܣ�˵��ø ��
��6���ڢۡ��ܡ��ݡ����Թ��У� ���Թ��������������п����ƻ�H2O2ø�ķ��ӽṹ���Ӷ�ʹ��ʧȥ���ԡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2015�츣����Դ��һ��ѧ��һ�ڶ����¿������Ծ��������棩 ���ͣ��ۺ���
H2O2������Ư�ף�Ҳ������ϴС���˿ڣ�ϸ���ڴ�л������Ҳ�����H2O2������ϸ���ж������ã��������й�������ø����ʹ��ֽ�Ϊ�����ʡ�
�������ͼ�е�ʵ�飬���ش��й����⣺��14�֣�
��1���ڢ١����Թ��У����Ȳ������ݵ����Թ� ����λʱ���ڲ��������������Թ� ��
��2�����Թܢ��У�ѡ�����ʸ�����ʵ�飬����Ϊ���ʸ����У���������
A.����������ࡡB.��������ø�ӷ� C.��ø������ࡡD.��������ø���һ���ǿ
(3)��ʵ����ȡ���ʸ���,Ѹ�ټ���Ͷ���Թܢ���,�����Ŀ���ǣ�������
A.��ֹ��������ø�ӷ�����������B.��ֹ��������ø�ֽ�
C.��ֹϸ���й�������ø���ͷš�D.ʹ��������ø��H2O2��ֽӴ�
��4���ȽϢٺ͢ں��Թܣ�˵��ø�� ��
��5���ȽϢںۢ͢ܢݢ��Թܣ�˵��ø ��
��6���ڢۡ��ܡ��ݡ����Թ��У� ���Թ��������������п����ƻ�H2O2ø�ķ��ӽṹ���Ӷ�ʹ��ʧȥ���ԡ�
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2011-2012ѧ�긣��ʡ����11���¿��������Ծ� ���ͣ��ۺ���
��5�֣�ϸ���ڴ�л�����У�������������⣬����ϸ���ж������ã��������й�������ø�Ĵ��ڿ��Խ���ֽ�Ϊ�������ʡ����������һ��ʵ�飬���ش��й����⣺
|
�Թܱ�� |
������ |
���� |
���� |
|
A |
2mL H2O2+2��FeCl3 |
30�� |
�ų��������� |
|
B |
2mL H2O2+2�θ�����ĥҺ |
30�� |
�ų��������� |
|
C |
2mL H2O2+2������ĸ�����ĥҺ |
��к���ȴ��30�� |
������ |
|
D |
2mL H2O2+2���䶳�ĸ�����ĥҺ |
0�� |
������ |
|
E |
2mL H2O2+2���˵�������Һ |
30�� |
������ |
��1��ʵ��B��E����˵��ø�Ĵ����þ���_______________�ص㡣
��2��ʵ��B��C����ͬ��ԭ����_______________________________________��
��3��ʵ��B����һ��ʱ����ٲ������ݣ������������е�øʧȥ���ԣ��������ǹ��������Ѿ������ֽ⣬�������ĸ�ԭ�����������ʵ����ơ���B�Թ��м��������� ���۲��������ݡ�����У���˵����ԭ��Ϊ ������ޣ���˵����ԭ�� Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com