
ijͬѧΪ��̽�����ȶԽ�ĸ���ƾ�����ø�ٷ�Ӧ���ʵ�Ӱ�죬���������ʵ�顣
��1��ʵ���þߣ��ձ���ע����4֧����ʿ�֡��ɽ�ĸ�ۡ����ǡ����ƺõ�pH�ֱ�Ϊ5��6��7��8�Ļ���Һ��
��2��ʵ�鲽�裺
���ÿ�ˮ������������Ϊ10%��������Һ100��������ȴ�����£�26 �棩����4�˸ɽ�ĸ�ۣ�10���Ӻ�
��_____________________________________________��
��_____________________________________________��
��_____________________________________________��
��_____________________________________________��
��3��ʵ���¼������2�������������������Ͳ�Ʊ������ĺ�����Ϊ������������Ҫ��ʱ�����±���
| pH | pH=5 | pH=6 | pH=7 | pH=8 |
| ʱ�䣨s�� | 8 | 7 | 6 | 7.5 |
��4��ʵ����ۣ�___________________________________________��
��5���ش����⣺ʵ�������ΪʲôҪ�ȵ�������Һ��ȴ���ټ����ĸ�ۣ�
��2����ȡ4֧ע�����ֱ���A��B��C��D�۷ֱ��ȡ2��������ĸ������Һ��A��B��C��D��Ӧ��ȡpHΪ5��6��7��8�Ļ���Һ�����÷�ʿ��ͿĨ����ͷ�Լ��йصط��ݹ۲첢��¼��Ͳ������2���������ʱ�䣨4��pHΪ7ʱ����ĸ���ķ����ٶ���죬������͵�pH����Ӱ���ĸ����ø�Ļ��ԣ�Ӱ�췢���ٶȣ�5����ֹ��ĸ��������ɱ���Է�ֹøʧȥ���ԡ�
����һ��ʵ������������⣬�ص㿼��ѧ�����ʵ����������������⣬�����ƺ�10%��������Һ��Ҫ�ø���������������������Һ�е�ϸ��������ɱ�����ͻ�Ӱ���ĸ�����͵Ľ��������Ŀ�У���������Һ������26 ��ʱ������ɽ�ĸ��4�ˣ�10���Ӻ�Ž�����һ��ʵ���ԭ�����ü��뵽������Һ�еĸɽ�ĸ��һ���ָ������Ĺ��̡���������ʵ�鲽����ο��𰸣�����ʵ����һ��Ҫע�⣺�ڼ���������Һ�ͽ�ĸ�Ļ��Һ�뻺��Һʱ��Ӧ�ȼ��뻺��Һ��ʹ��ĸ��һ�����Թܾʹ���һ����pH�����£������ڲ�ͬʵ�������±Ƚϡ�


 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ����������� ���ͣ�071
ijͬѧΪ��̽�����ȶԽ�ĸ���ƾ�����ø�ٷ�Ӧ�ٶȵ�Ӱ�죬���������ʵ�顣
��1��ʵ���þߣ��ձ���ע����4֧����ʿ�֡��ɽ�ĸ�ۡ����ǡ����ƺõ�pH�ֱ�Ϊ5��6��7��8�Ļ���Һ��
��2��ʵ�鲽�裺
���ÿ�ˮ������������Ϊ10����������Һ100��������ȴ�����£�26�棩����4�˸ɽ�ĸ�ۣ�10���Ӻ�
��______________________________��
��______________________________��
��______________________________��
��______________________________��
��3��ʵ���¼������2�������������������Ͳ�Ʊ������ĺ�����Ϊ������������Ҫ��ʱ�����±���
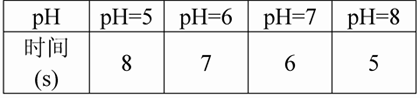
��4��ʵ����ۣ�______________________________
��5��ʵ�������ΪʲôҪ�ȵ�������Һ��ȴ���ټ����ĸ�ۣ�
___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�� ���ͣ�043
ijͬѧΪ��̽�����ȶԽ�ĸ���ƾ�����ø�ٷ�Ӧ�ٶȵ�Ӱ�죬���������ʵ�顣
��1��ʵ���þߣ��ձ���ע����4֧����ʿ�֡��ɽ�ĸ�ۡ����ǡ����ƺõ�pH�ֱ�Ϊ5��6��7��8�Ļ���Һ��
��2��ʵ�鲽�裺
���ÿ�ˮ������������Ϊ10����������Һ100��������ȴ�����£�26�棩����4�˸ɽ�ĸ�ۣ�10���Ӻ�
��______________________________��
��______________________________��
��______________________________��
��______________________________��
��3��ʵ���¼������2�������������������Ͳ�Ʊ������ĺ�����Ϊ������������Ҫ��ʱ�����±���
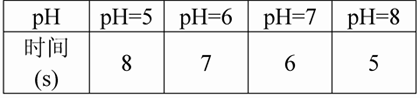
��4��ʵ����ۣ�______________________________
��5��ʵ�������ΪʲôҪ�ȵ�������Һ��ȴ���ټ����ĸ�ۣ�
___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��2013-2014ѧ�꽭��ʡ�߶���ѧ�ڿ�ѧ���������Ծ��������棩 ���ͣ��ۺ���
��7�֣�ʵ��̽����ijͬѧΪ��̽�����ȶԽ�ĸ���ƾ����͵�ø�ٷ�Ӧ���ʵ�Ӱ�죬���������ʵ�飺
��1��ʵ���þߣ��ձ���ע���� 4 ֧����ʿ�֡��ɽ�ĸ�ۡ������ǡ����ƺõ�pH�ֱ�Ϊ5��6��7��8�Ļ���Һ��
��2��ʵ�鲽�裺
���ÿ�ˮ������������Ϊ 10% ����������Һ 100mL ����ȴ�����£� 26�� ������ 4g �ɽ�ĸ�ۣ���Ӧ10min ��
��_______________________________________________________
��__________________________________________________________
��____________________________________________________________
��____________________________________________________________
��3��ʵ���¼������ 2mL ���������������Ͳ�Ʊ������ĺ�������ʾΪ������������Ҫ��ʱ�����±���
|
pH |
pH=5 |
pH=6 |
pH=7 |
pH=8 |
|
ʱ�� �� s �� |
8 |
7 |
6 |
7.5 |
��4��ʵ����ۣ�________________________________________________________��
��5���ش����⣺ʵ�������ΪʲôҪ�ȵ���������Һ��ȴ���ټ����ĸ�ۣ�
___________________________________________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com