
��ͼ��ʾ����ơ�Ƶķ�����
��1��ΪʲôҪ��ԭ�Ϸ����ˮ�л�ϣ�___________________________________��
��2��Ϊʲô�ڻ������ȴ��Űѽ�ĸ���ӽ�ȥ��________________________________��
��3��ΪʲôҪ�ڷ����ϼӸ��ӣ�___________________________________��
��4��д����ĸ��ϸ�������е��������̵Ļ�ѧ����ʽ��____________________________��
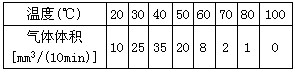
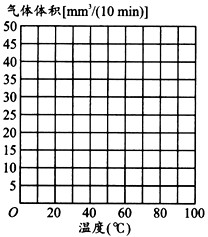
��5����ѧ���о��˷��ͳ����ǵ����ã����Ƿ����¶ȱ仯�����������Ӱ�죬������20 �����ռ�10�����ڷų�������������Ժ��ڲ�ͬ�¶����ظ��������IJ��������ǵļ�¼���±���
| �¶ȣ��棩 | ���������mm3/10 min�� |
| 20 | 10 |
| 30 | 25 |
| 40 | 35 |
| 50 | 20 |
| 60 | 8 |
| 70 | 2 |
| 80 | 1 |
| 100 | 0 |
������������ͼ�У����������ݻ���һ��ƽ�������ߡ�

��6����������Ϊʲô��������״��___________________________________________��
��7��ơ�Ƶķ�ζ��Ҫȡ���������õĽ�ĸ���ꡣij�Ƴ��ľ���������30���꣬��ֳ����ǧ�������ζ�������ꡣ����Ҫԭ����___________________________________��
��1��ɱ��ϸ������2����ֹ����ɱ����ĸ������3����ĸ������ơ�Ƶķ������������ͣ���ֹ�������루4��C6H12O6![]() 2C2H5OH+2CO2+������C6H12O6+6O2+6H2O
2C2H5OH+2CO2+������C6H12O6+6O2+6H2O![]() 6CO2+12H2O+����
6CO2+12H2O+����
��5��

��6����ĸ�����ͻ������������������ø�Ĵ��²��ܽ��У�ø�Ļ������¶��йأ����¶ȳ���40 ��ʱ��ø�Ļ����½��������»�ʹø�ṹ���ƻ����Ӷ�ʹøʧȥ���ԣ�7����ĸ����������ֳ
�����֪ʶ����������͵Ļ���������ע�������ĸ���������÷�Ӧʽ�����������Ŀ��ƺͽ�ĸ������ֳ��ʽ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ���������� ��Դ��2004ȫ����ʡ�и߿�ģ�������ࡤ���� ���ͣ�022
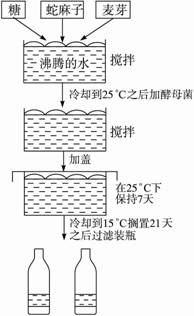
��ͼ��ʾ����ơ�Ƶķ������̣���ش�

��1��ΪʲôҪ��ԭ�ϷŽ���ˮ�л�ϣ�________��
��2��ΪʲôҪ�ڻ������ȴ��Űѽ�ĸ�ӽ�ȥ��________��
��3����д����ĸ�������е��������̵Ļ�ѧ��Ӧ����ʽ��
��4���о��߷����¶ȵı仯�����������Ӱ�죬���ڲ�ͬ�¶��²ⶨ����IJ������±����������з���ֽ�ϣ��ñ������ݣ�����������ͼ��


��5���������������Ϊʲô����������״��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ�������ɽ��������߿�����ڶ��֣� ���ͣ�043
��ͼ��ʾ����ơ�Ƶķ������ʣ�
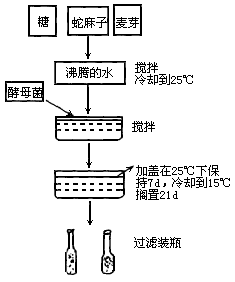
��1��ΪʲôҪ��ԭ�Ϸ����ˮ�л�ϣ�________��
��2��ΪʲôҪ�ڻ������ȴ��Űѽ�ĸ���ӽ�ȥ��________��
��3��ΪʲôҪ�ڷ����ϼӸ��ӣ�________��
��4��д����ĸ��ϸ�������е��������̵Ļ�ѧ����ʽ________��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ���Ƹ���ѧ���߶�����²ᣩ����ĩ���Ծ� ���ͣ�071
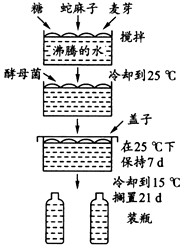
��ͼ��ʾ����ơ�Ƶķ������̡���ͼ�ش��������⣺

��1��ΪʲôҪ��ԭ�Ϸ����ˮ�л�ϣ�
��2��ΪʲôҪ�ڻ������ȴ��Űѽ�ĸ���ӽ�ȥ��
��3��ΪʲôҪ�ڷ����ϼӸ��ӣ�
��4��д����ĸ��ϸ�������е��������̵Ļ�ѧ��Ӧʽ��
��5��ơ�Ƶķ�ζ��Ҫȡ���������õĽ�ĸ���ꡣij�Ƴ��ľ������30���꣬��ֳ��ǧ�������ζ�������ꡣ����Ҫԭ����ʲô��
�鿴�𰸺ͽ���>>
��Ŀ���������� ��Դ��ͬ���� ���ͣ���ͼ�����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com