
��7�֣�����ѧ��⣬�����к�̼���83��85%��������15��17%�������ж�����Ԫ��п��ͭ���������ȡ�С��ͬѧΪ�����Լ�⵰���е�ijЩ�ɷ��Լ�������������ʵ��̽������ش�������⣺
��1�����ڵ��ǵ������У�����Ϊ�����һ���ǣ� ��
| A�����ơ�п��ͭ������������Ԫ�� |
| B������������л������ɵĻ���� |
| C�����DZ����зۣ�θ�����߷��ã���ֹʹ������θ������Ч�� |
| D�����Լӹ�������ơ���������ƵȲ�Ʒ�����Ϊ�� |

��1��A
��2��ȡ�����������Թ��У������еμ�������ϡ���ᣬ��������ð�����Ҹ�������ʹʯ��ˮ����ǣ�˵�����ǵijɷֺ���̼���
��3��83.3%
���������������1��A������п��ͭ������������Ԫ�أ������ڳ���Ԫ�أ�����B�������к�̼��ƣ���������������л����ȷ��C�������к�̼�������θҺ�е����ᷴӦ�����Ե��DZ����зۣ�θ�����߷��ã���ֹʹ������θ������Ч������ȷ��D�������к�̼��ƣ����Լӹ�������ơ���������ƵȲ�Ʒ�����Ϊ������ȷ����ѡA
��2��̼���εļ��飺ҩƷ��ϡ����ͳ���ʯ��ˮ��ʵ�鷽���ǣ�ȡ�����������Թ��У������еμ�������ϡ���ᣬ��������ð�����Ҹ�������ʹʯ��ˮ����ǣ�˵�����ǵijɷֺ���̼���
��3�������к�̼����������ᷢ����Ӧ��CaCO3+2HCl==CaCl2+H2O+CO2������Ӧ�����˶�����̼���壬���Ը��������غ㶨�ɣ������ж����ɶ�����̼������=6g+50g-53.8g=2.2g���ٸ��ݷ���ʽ��CO2��CaCO3��������ϵ��������CaCO3����������һ�����㵰����̼��Ƶ���������
�⣺��̼��Ƶ�����Ϊx����
CaCO3 + 2HCl=CaCl2 + H2O + CO2��
100 44
x 2.2g
100/x=44/2.2g
x=5g
�� ̼��Ƶ���������=5g/6g��100%=83.3%
���㣺��ѧ�����̼���εļ��飬���ݷ���ʽ����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��5�֣�����20 g��Fe2O3Ϊ80%�ij�����ʯ�����뵽150 gϡ�����У�ǡ����ȫ��Ӧ������ʯ�е����ʶ�������ˮ���Ҳ���ϡ���ᷴӦ����(����������һλС��)
��1��������ʯ��Fe2O3������Ϊ g�� Fe2O3����Ԫ�ص�����Ϊ g��
��2��ϡ���������ʵ�����������
��3��ǡ����ȫ��Ӧ��������Һ�����ʵ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�Ϊ�ⶨNaCl��Na2SO4����������NaCl��������������ѧС��ͬѧ��������ʵ����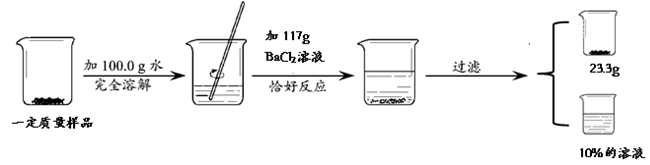
�ɴ˲��ԭ����������NaCl�����������Ƕ��٣�����֪��Na2SO4 + BaCl2 ===BaSO4�� + 2NaCl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��9�֣�ij��Һ�п��ܺ���̼���ơ��Ȼ��ء������ơ������е�һ�ֻ��֣�Ϊ�ⶨ����ɣ���������ʵ�飺
ȡ��20g���Ⱥ���μ����Ȼ�����Һ��ϡ���ᣬ����������������ʱ��Ĺ�ϵ��ͼ��ʾ��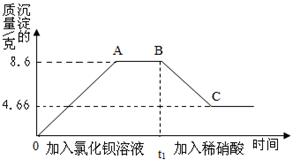
��1��ʵ���У�AB֮��ͼ������Ӧ����Һ�е�����һ������ ����
��2��BC�γ������ٵ�ԭ���� �����û�ѧ����ʽ��ʾ����
��3��������ʵ���֪��ԭ��Һ��һ�������� ���������� ��
��4����ԭ��Һ�������Ƶ�����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��5�֣�ijУ����С���ͬѧ�ڲⶨ��MgCl2��NaCl��ɵĹ�����������ʱ������������ʵ�顣ȡ20�˹������200����Һ��ƽ���ֳ��ķݣ�Ȼ��ֱ����һ������������NaOH��Һ������ʵ�����ݼ��±���
| | ʵ��һ | ʵ��� | ʵ���� | ʵ���� |
| ����������Һ������/g | 50 | 50 | 50 | 50 |
| ����NaOH��Һ������/g | 10 | 20 | 30 | 40 |
| ���ɳ���������/g | 1 | m | 2.9 | 2.9 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
(7��) ��ҵ�ϳ���ϡ���ᴦ������Ʒ��������⣬�ݴ���ش��������⣺
��1����ȥ����Ĺ����л����Կ�����һ��ʵ��������______________________________��
��2�����ϡ��������̫��ͻῴ�������ݲ������û�ѧ����ʽ˵��ԭ��
____________________________________________��
��3����ҵ������30ǧ��������������Ϊ7.3%��ϡ���ᣬ��ij����Ʒ���������ǡ����ȫ��Ӧ����Ӧ����Һ������������������ȷ��С�����һλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��6�֣�����·�����У����������з�Ӧʵ�ָֹ�Խӣ�Fe2O3 + 2Al ���� 2Fe + Al2O3 ������������Ҫ56kg���������������۵������Ƕ��٣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��4�֣���100g������3.2%�������ۣ����ʲ����뷴Ӧ��������������Ϊ10%��ϡ����ǡ����ȫ��Ӧ�����������������ĵ�ϡ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��3�֣�ú�������ǽ���ת��Ϊ��ȼ������Ĺ��̣���Ҫ��ӦΪ��C + H2O  CO + H2��
CO + H2��
������1 t��������������̼��������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com