
��������пո�ÿ��1�֣���10�֣�
��1����������������NH4+ ��CuSO4 ��S8 ��SO42-��Ca(OH)2�������ܱ�ʾ�ε��� ������ţ���ͬ���������ܱ�ʾ���ӵ��� ���ٺ͢����������ɵĻ����ﻯѧʽ �����������������̼���� ����Һ��
��ѧ�����ȷ�������ﻯѧ��Ϣ�����û�ѧ������ա�
�ٵؿ��к����ߵڶ�λ�ķǽ���Ԫ�� ��þԭ�ӽṹʾ��ͼ
�� ��ʯ���и���+2�� ������л���
�� �����³�Һ̬�Ľ��� ��n��̼�������

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
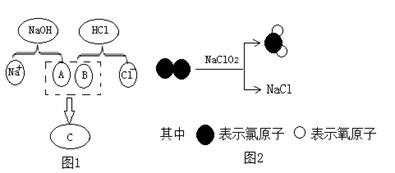
��84������Һ����Ч�ɷ�NaClO����Ԫ�صĻ��ϼ�Ϊ ��Cl2��������ˮ����������ʵ�����Ʒ�ΪMnO2��4HCl(Ũ) X��Cl2����H2O,��X�Ļ�ѧʽΪ ��
X��Cl2����H2O,��X�Ļ�ѧʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��8�֣���ѧ�����ǵ�����ʵ����ϵ���У���������ѧ�Ļ�ѧ֪ʶ�ش����м���С���⣺
��1��ũ���ô�ĥ�ɾ��ķ�������ʢ����ȡũҩ��������Һ����ԭ������ͭ��Һʱ�������к�ɫ���ʳ��֣���ӽ������˳��ĽǶ�˵�����ָ������ԭ��
��2��ʩ��NH4HCO3�������ʱ���� ���������������磬������ϰϰ�Ļƻ裩
��3�����ǿ�����ϡ�ͺ��������Һ��ȥ�����ϵİ߰��⼣��ԭ���ǣ��û�ѧ����ʽ˵����
��4����ʱ��ʹ�õ���ˮ�������ڱ��ϻ���һ���ɫ��ˮ�����γ�ˮ������Ҫ�ɷ���̼��ƣ�����д���γ�ˮ����Ҫ�ɷֵĻ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣������������ڿ�ʯ�У����������ڳ�����Fe2O3����������Fe3O4����������̼����������ѧʽΪ ����ͭ�����ڻ�ͭ��CuFeS2������ͭ��Cu2S���������Ǹ����ۣ���������Ϊ ���ȡ����������ڹ�ҵ��������Ӧ�Ļ�ѧ����ʽ�� ��Fe3O4������Ͽɿ���Fe2O3��FeO������������������ᷴӦ����һ��ʹ��Һ�ʻ�ɫ�����ʺ�һ��ʹ��Һ��dz��ɫ�����ʣ�����һ����ѧ����ʽ��ʾ�÷�Ӧ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��6�֣�д�����з�Ӧ�Ļ�ѧ����ʽ��
��1�����ǻ��ý����������е�����ȴ�н�ǿ�Ŀ���ʴ�ԣ�ԭ����
��2����ҵ����һ����̼��ԭ��������Ҫ�ɷ���Fe2O3�������Ļ�ѧ����ʽ��
��3��Ϊ��֤ij�������ʼ�����Ҫ�ɷ�Ϊ���ۣ�ijͬѧ��ϡ���������������У��۲쵽���������ɣ�����������ŵ���һ�ɳ���������ζ��ԭ�����������г��������������������FeS������������ϡ���ᷢ���˸��ֽⷴӦ���������������ɣ���д���÷�Ӧ�Ļ�ѧ����ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�÷��ź�������գ�
��2�����������________����3����������________
��+3�۵���Ԫ��_________���ܳ����õ����Ե�ζƷ�е���_________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�����귢ׯ�ڡ��������ѧ���ݵġ��磨�ŵ磩ʱ�������е���Ҫ�ɷֵ���������ֱ�ӻ�������һ���������壬һ������������������е�����������ɶ����������壬������������ˮ���������һ�����������ɵ���������ˮ���䵽��أ��������еĿ������������ɿ����������Σ�Ϊֲ���ṩ�˵��ʡ��ӵ����������Ĺ����У������ķ�Ӧ�Ļ�ѧ����ʽΪ �� �� ���������ڻ��Ϸ�Ӧ���� ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧԴ���������������������������ж��̺��Ż�ѧ֪ʶ��������ա�
��1�������ҹ����õĿ��ٷ�չ�����ٵ���Դ��CO2����ѹ���������ء�̫���ܹ��ˮ�ɽ�̫����ֱ�Ӹ�Чת��Ϊ�ྻ����������������Դ������������Դ�� ��
��2��ȱ��������״���״�Ӱ������������������ʳ���м��������������Ԫ����������������⡣�����÷�Ӧ5KI+X+3H2SO4��3K2SO4+3I2+3H2O��X��ʾ����أ�������ʳ�����Ƿ�ӵ⣬�����صĻ�ѧʽ��
��3����ͥ�ڳ��˹����������¹��߷����Ż�������ֱ�ӵ�������ù��Ǹ��������ԭ����
��4�����ڶ�����ȫ���ѳ�Ϊ���ܻ�ӭ��ʳƷ���±��Ƕ�����������Ҫ�ɷֵ�ƽ������������
| �ɷ� | ˮ | ������ | ��֬ | ���� | �� | �� | �� |
| ��������/% | 89.3 | 4.7 | 1.3 | 2.8 | 0.24 | 0.064 | 1.4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ѧ�����������������ʣ�Ҳ�ܱ������۵ı仯��
��1�����з����ܱ�ʾ2����ԭ�ӵ��� ����д��ţ���ͬ�����ܱ�ʾ����ˮ�Ļ�ѧ���ʵ����� ��
| A��H2 | B��2H | C��H2O | D��2H2O |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com