CO
2�������Ʊ�̼�����ϣ��������������У�ijͬѧ�������ʵ�飬�ⶨijƷ��̼�������е�CO
2�����
ʵ�鷽��һ��
�ٽ�250mLƿװ̼�����Ϸ��ڱ������䶳���պý����
�ڽ����ϴӱ�����ȡ����Ѹ�ټ�����������Ϊ50%NaOH��Һ5mL����ת����ƿ��������Ȼ����ûָ������£����ⶨ��
�۳Ƶ�װ��C������Ϊx g����ͼ1����ʵ��װ�ã�ȡ50mL����Һ���������У��رտ���a���������ٵĻ���������������ע��ϡ���ᣬ�����ٲ������ݣ��ر������ٵĻ�����
�ܴ���a����װ���й��������һ��ʱ�����Cװ������Ϊy g��
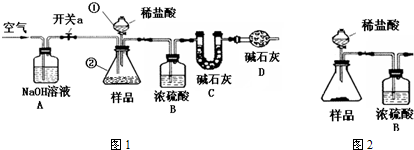
ע��ʵ���и�����Һ���ܶȶ���Ϊ1g/mL����ʯ����CaO��NaOH�Ĺ������
��1������װ���У������ٵ�����Ϊ
��Һ©��
��Һ©��
�������ڵ�����Ϊ
��ƿ
��ƿ
��
��2��װ��A��Ŀ����
���տ����еĶ�����̼��ˮ
���տ����еĶ�����̼��ˮ
��װ��D��Ŀ����
��ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ����
��ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ����
��
��3��������䶳̼�����ϵ�Ŀ����
��ֹ������̼�ݳ�
��ֹ������̼�ݳ�
��������з�Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
������NaOH��Һ��Ŀ����
���ն�����̼
���ն�����̼
��
��4������ܹ��������Ŀ����
�����ɵĶ�����̼ȫ������װ��C��
�����ɵĶ�����̼ȫ������װ��C��
��
��5�����������ṩ�����ݼ����Ʒ��̼��������CO
2�ĺ���Ϊ
20��y-x��
20��y-x��
g/L��
��6����û��Bװ�ã�����CO
2������
ƫ��
ƫ��
�����ƫ��ƫС����
ʵ�鷽������
�٢��뷽��һ��ͬ���۰�ͼ2����ʵ��װ�ã��Ƶ�����װ�ã���ҩƷ��������������ע������ϡ���ᣬ���10s������������װ�õ�������ֱ������װ�õ���������Ϊֹ��
��1��ע������ϡ��������Ļ�ѧ��Ӧ����ʽΪ
Na2CO3+2HCl�T2NaCl+H2O+CO2��
Na2CO3+2HCl�T2NaCl+H2O+CO2��
��
��2����ʵ���г����������ٽ���
3
3
�Σ�
��3��װ��B��Ŀ����
���������е�ˮ��
���������е�ˮ��
��
��4����ͬѧ����÷������CO
2������ƫС����ͬѧ�����ɳ��˿����ǿ�����ˮ������ʵ������Ӱ���⣬��������
��ƿ�ڲ����ж�����̼
��ƿ�ڲ����ж�����̼
��






