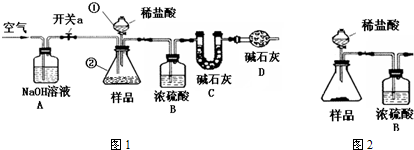
�⣺��1�������ٵ�����Ϊ��Һ©���������ڵ�����Ϊ��ƿ��
��2��������ˮ�Ͷ�����̼��ʵ�������Ӱ�죬����Aװ�õ����������տ����еĶ�����̼��ˮ��Dװ�õ������Ƿ�ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3��������̼���ܽ�����¶ȵĽ��Ͷ���������䶳̼�����ϵ�Ŀ���Ƿ�ֹ������̼�ݳ��������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa
2CO
3+2HCl�T2NaCl+H
2O+CO
2��������������Һ�����ն�����̼���壬����NaOH��Һ��Ŀ�������ն�����̼��
��4�����������Ŀ���������ɵĶ�����̼ȫ������װ��C�У�
��5��Cװ���������Ӿ��Ƕ�����̼��������
���ɶ�����̼������=yg-xg
��Ʒ��̼��������CO
2���=

=20��y-x��g/L
�𣺴�Ʒ��̼��������CO
2�ĺ���Ϊ20��y-x��g/L��
��6��û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO
2������ƫ��
ʵ�鷽��������1�������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ��Ӧ����ʽΪNa
2CO
3+2HCl�T2NaCl+H
2O+CO
2����
��2����ʵ���г����������ٽ���3�Σ�
��3��Ũ������������ԣ����������е�ˮ�֣�
��4����ƿ�ڲ����ж�����̼�����CO
2������ƫС��ʹ�ã�
�ʴ�Ϊ����1����Һ©������ƿ��
��2�����տ����еĶ�����̼��ˮ����ֹ�����еĶ�����̼��ˮ���룬Ӱ��ʵ������
��3����ֹ������̼�ݳ���Na
2CO
3+2HCl�T2NaCl+H
2O+CO
2�������ն�����̼��
��4�������ɵĶ�����̼ȫ������װ��C�У�
��5��20��y-x����
��6��ƫ��
ʵ�鷽��������1��Na
2CO
3+2HCl�T2NaCl+H
2O+CO
2������2��3����3�����������е�ˮ�֣���4����ƿ�ڲ����ж�����̼��
��������1�����ݳ������������ƽ��н��
��2�����ݿ�����ˮ�Ͷ�����̼��ʵ�������Ӱ����н��
��3�����ݶ�����̼���ܽ�����¶ȵĽ��Ͷ����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼�Լ�����������Һ�����ն�����̼������н��
��4�����ݹ��������Ŀ���������ɵĶ�����̼ȫ������װ��C�н��н��
��5������Cװ���������Ӿ��Ƕ�����̼���������н��
��6������û��Bװ�ÿ����еĶ�����̼��ˮ�־ͻ���룬ʹ�ò��CO
2������ƫ����н��
ʵ�鷽��������1�����������̼���Ʒ�Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��2������ʵ���г����������н��
��3������Ũ������������Խ��н��
��4��������ƿ�ڲ����ж�����̼���н��
����������Ͷ���ѧ�Ļ�ѧ֪ʶ�����ʵ���ɡ�̼���ƺ�ϡ����ķ�Ӧ�����ݻ�ѧ����ʽ�ļ�������ʵĴ��Ƚ����˿��飬Ҫ����������Щ֪ʶ����д��ѧ����ʽһ��Ҫȷ����


 =20��y-x��g/L
=20��y-x��g/L