
����Ŀ�������г��õ�Ħ������̼��Ʒ�ĩ���ú�̼���90%��ʯ��ʯ��Ϊԭ�ϣ��Ƶ�̼��Ʒ�ĩ�����ʲ����뷴Ӧ���������������£�

�� �����Ƶ�������_______��
�� ʯ��������__________��
A������Һ B������Һ C����Һ
�� ����II�����IJ���������______________��
�� ��д�����������з������Ϸ�Ӧ�Ļ�ѧ����ʽ___________________��
�� �����ϵõ�̼�������______��ѡ�����������������ʯ��ʯ��̼��Ƶ�������
�� ���������������9mol������̼�������ĸ�ʯ��ʯ������Ϊ����g��__________�����ݻ�ѧ����ʽ��ʽ���㣩
���𰸡���ʯ�� A ���� CaO+H2O![]() Ca(OH)2 = �⣺�����ĵ�CaCO3�����ʵ���Ϊxmol
Ca(OH)2 = �⣺�����ĵ�CaCO3�����ʵ���Ϊxmol
CaCO3![]() CaO + CO2�� ��1�֣�
CaO + CO2�� ��1�֣�
1 1
x 9
![]() ��1�֣�
��1�֣�
x=9 ��1�֣�
9mol��100g/mol= 900g ��1�֣�
900/90%=1000g ��1�֣�
�����ĸ�ʯ��ʯ������Ϊ1000g��
��������
�ٸ��������Ƶ���������ʯ�ҽ��
�ڸ�������Һ������Һ�Ķ�����з����ش�
�۹����ǽ�������Һ��Ĺ�����������һ�ַ�����
�ܸ�����������ˮ��Ӧ�������������ƽ��
�ݸ��������غ㶨�ɻ�ѧ��Ӧǰ��Ԫ�ص��������
���ݶ�����̼���������÷�Ӧ�Ļ�ѧ����ʽ���̼��Ƶ�������
�⣺�������Ƶ���������ʯ�ң�
�ڹ���С������Һ���γɵIJ���һ�����ȶ��Ļ����ͽ�����Һ��Һ����Һ���γɵIJ���һ�����ȶ��Ļ����ͽ�����Һ���������ǹ���С������Һ���γɵĹ�Ϊ����Һ��
�۹����ǰѲ�������Һ�Ĺ����Һ��ֿ���һ�ַ�������ķ���������Ӧ�ء��еĻ�����������ɵ�̼��ƹ��壬���ù��˵ķ�����
����������ˮ��Ӧ�������������ƣ��������Ϸ�Ӧ�Ļ�ѧ����ʽΪ��CaO+H2O�TCa��OH��2��
�ݸ��������غ㶨�ɻ�ѧ��Ӧǰ��Ԫ�ص�����䣬�����ϵõ�̼�����������ʯ��ʯ��̼��Ƶ�������
�������ĵ�CaCO3�����ʵ���Ϊxmol
CaCO3![]() CaO+CO2��
CaO+CO2��
1 1
x 9mol
![]()
x=9mol
9mol��100g/mol=900g
![]() =1000g
=1000g
�𰸣�
����ʯ�ң�
��A��
�۹��ˣ�
��CaO+H2O�TCa��OH��2��
��=��
�����ĸ�ʯ��ʯ������Ϊ1000g��


 ��ĩ�óɼ�ϵ�д�
��ĩ�óɼ�ϵ�д� 99��1������ĩ��ѵ��ϵ�д�
99��1������ĩ��ѵ��ϵ�д� ��ǿ��У��ĩ���100��ϵ�д�
��ǿ��У��ĩ���100��ϵ�д� �óɼ�1��1��ĩ���100��ϵ�д�
�óɼ�1��1��ĩ���100��ϵ�д� ��״Ԫ���źþ�ϵ�д�
��״Ԫ���źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
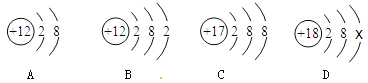
����Ŀ����ͼ��A��B��C��D���������ӵĽṹʾ��ͼ��
��ش��������⣺
��1��ͼ��A��B��C��D����_______��Ԫ�ص����ӣ�
��2��A��B��C��D���������У����߱��ȶ��ṹ����_________������ţ���
��3��D��x��________��
��4��A��C�γɻ�����Ļ�ѧʽ��___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Ԥ������H1N1���е���Ҫ��ʩ֮һ��
��1��ij����Ҫ��2875��������������Ϊ3���Ĺ���������Һ����������Ҫ��________����������������Ϊ15%�Ĺ���������Һ�����ơ���15���Ĺ���������Һ�ܶ�Ϊ1��15g/cm3��
��2������H1N1���в�����ֱ��Ϊ0.08��0.12�ף����в����ķ�ĭֱ��һ��Ϊ1��10�ס����õ����ֿ��ֹ��˿����£�����ͨ16��ɴ��������100�����ң��ڵ����IJ�������10�����ң���N95רҵ������0��1�����ҡ����������ڷ��ؼ���H1N1�����и���Ч����___(�����)�����ֵ������ǹ��ˣ��ɴ���Թ����к��µ���ʶ?____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ����������֮���ת����ϵ������һ����ʾ��������֮���ܷ�����Ӧ������������ʾһ�����ʿ���ת��Ϊ��һ�����ʡ�����A��B��C��D��E��F�������ʣ�����FΪϡ���ᣬE��D��C�ж���̼Ԫ�أ�BΪ����ɫ��A�������ڽ������ش���������(����������)��
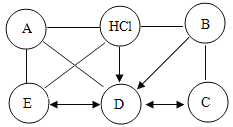
��1��Eת��ΪD��Ӧ�Ļ�ѧ����ʽΪ_____��
��2��B���������ᷴӦ��������_____��
��3��д��ͼ��A��B��C��D��E��F��������(������)�Ļ�ѧʽ_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��̼������ͭǡ����ȫ��Ӧ���й����ı仯����ͼ��������ȷ����
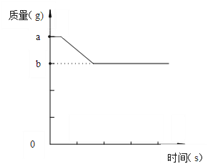
A. ��a-b����ʾ��Ӧ���ĵ�̼������
B. ��a-b����ʾ��Ӧǰ���������Ԫ�ص�����
C. b��ʾ���ɵ�ͭ������
D. b��ʾ���ɵĶ�����̼������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ˮ��⣬��Ӧ�Ļ�ѧ����ʽΪ__________���÷�Ӧ�Ļ���������_____����������ʯ��ʯ����Ӧ�Ļ�ѧ����ʽΪ______���÷�Ӧ�Ļ���������_____������������ȼ�յĻ�ѧ����ʽΪ______���÷�Ӧ�Ļ���������______��ľ̿��ԭ����ͭ�Ļ�ѧ����ʽΪ________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ֺͻ�ѧ������գ�
��3��ˮ����_____��
��2����ԭ��_____��
����3�۵���Ԫ��_____��
��������_____��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ͼ��A��B��C�ֱ��ʾ���ֲ�ͬ�Ĺ������ʣ������ᾧˮ�������ܽ��������ͼ��ʾ����ش��������⣺
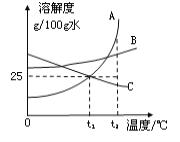
�� t1��ʱ��100gˮ������ܽ����A______g��
�� t2��ʱ�����ֹ��������ܽ����С�����˳����__________��
�� t1��ʱ����30g����C�ӵ�50gˮ�У���ֽ����������Һ������Ϊ_________g��
�� д��һ�ֽ�����B�IJ�������Һת��Ϊ������Һ�ķ���_______________��
�� ����������������Ϊ16����A��Һ������ˮ������l00g������������Ϊ10����A��Һ��������16����A��Һ������ˮ��������Ϊ_____________��
�� һ���¶��£���ͼ4�ձ��м���һ������ˮ����ͼ5��ʾ��������Һ��ԭ��Һ��ȣ��ж�һ����ȷ����_______��ѡ���ţ���

A��������Һ�DZ�����Һ B�����ʵ������������
C�����ʵ��������� D�����ʵ��ܽ�ȱ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������д����л�ѧ����ѧ������������ء�
��1��ij���ɰ�װ�����õĸ��������ʯ�ң����û�ѧ����ʽ��ʾ��ԭ��_____��
��2��ɭ�ֻ���ʱ����������һ���������Ŀ����_____��
��3�����dz���ϴ�Ӽ�ϴ�Ӳ;��ϵ����ۣ�������Ϊϴ�Ӽ�����_____���ܣ�������ϴ������_____����
��4������̿���ھ��к�ǿ��_____�ԣ���������������ڵ���ζ��
��5���ó����������Ļ�ѧ����ʽΪ_____�����������������Ͻ𣬴�����Ͽ������ߵ�_____��ͬ��
��6������Ʒ��ʴ��ʵ��������������е�_____�����˻�ѧ��Ӧ����ֹ������ʴ��һ�ַ�����_____������������ʴ��ԭ����_____���û�ѧ����ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com